
What is the name of \[SO_{4}^{2-}\] ion?
a.) Persulfate
b.) Sulfate
c.) Sulfite
d.) Hyposulfite
e.) Sulfide
Answer
559.5k+ views
Hint: -Ate is the suffix used for the ions where the central atom is in its highest oxidation state and per suffix is used when oxygen-oxygen bond is present in the ion. Also we must remember the number of electrons in sulfur atom’s valence shells are 6, so the highest oxidation number it can show is 6.
Complete step by step answer:
$SO_{4}^{2-}\to $ Here one sulfur atom is attached to 4 oxygen atoms. So it’s possible structure can be as shown below:
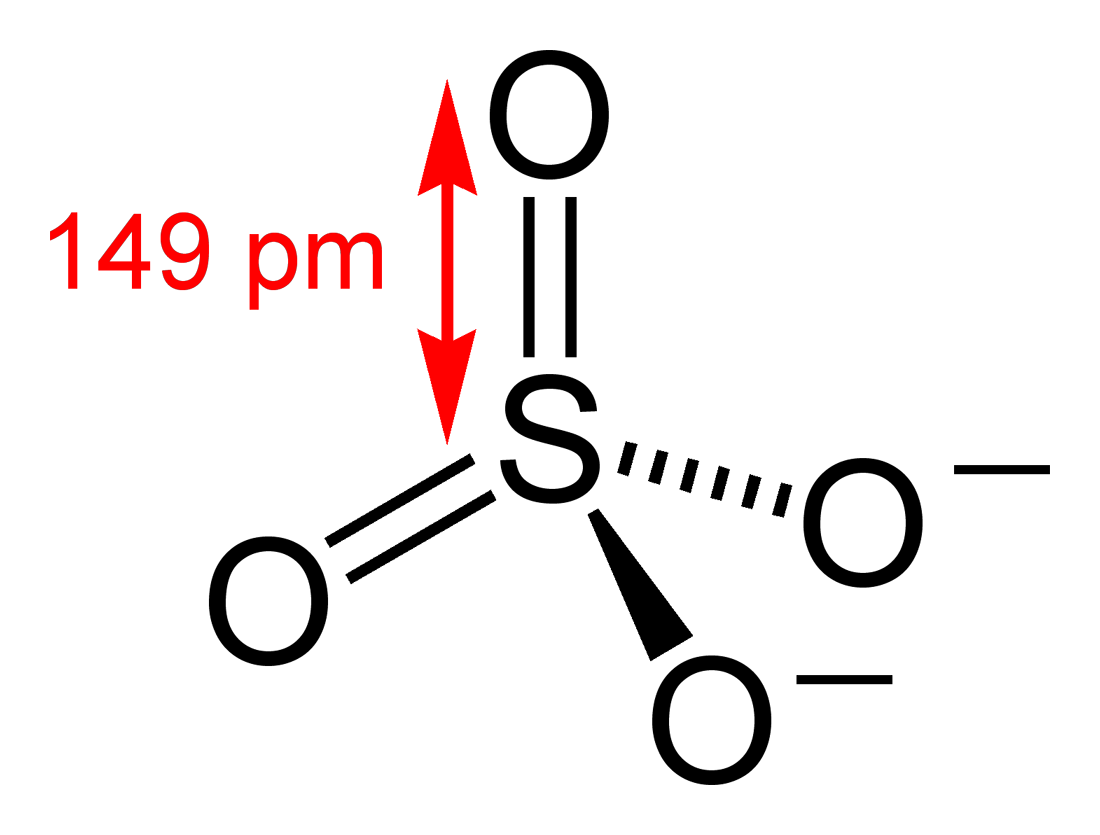
- In this structure two oxygen atoms have formed multiple bonds with sulfur and two oxygen atoms have formed a single bond. As we know oxygen atoms are more electronegative than sulfur and because of that sulfur will bear +1 positive charge for each bond it has formed with oxygen.
Total bonds formed with oxygen over here = 6
Total formal positive charge on Sulfur = +6
Oxidation number of sulfur= +6
As oxidation number of sulfur is its highest possible oxidation number and no oxygen-oxygen bond is present over here so the name of this ion will be Sulfate.
Persulfate ion: ${{S}_{2}}O_{8}^{2-}$
Sulfite ion: $SO_{3}^{2-}$
Hyposulfite ion: ${{S}_{2}}O_{3}^{2-}$
Sulfide ion: ${{S}^{2-}}$
Additional Information : Sulfates occur as microscopic particles (aerosols) resulting from fossil and biomass combustion. They increase the acidity of the atmosphere and form acid rain.
Note: Sulfate ion present in general along with phosphate for qualitative analysis of ions. Confirmatory tests of sulfate ion are as follows:
a.) Aqueous solution or sodium carbonate extract of the salt acidified with acetic acid in addition to barium chloride gives a white precipitate of barium sulfate insoluble in conc. $HCl$ or conc. $HN{{O}_{3}}$ .
b.) Sulfate ions give white precipitate of lead sulphate when aqueous solution or sodium carbonate extract neutralised with acetic acid is treated with lead acetate solution.
Complete step by step answer:
$SO_{4}^{2-}\to $ Here one sulfur atom is attached to 4 oxygen atoms. So it’s possible structure can be as shown below:

- In this structure two oxygen atoms have formed multiple bonds with sulfur and two oxygen atoms have formed a single bond. As we know oxygen atoms are more electronegative than sulfur and because of that sulfur will bear +1 positive charge for each bond it has formed with oxygen.
Total bonds formed with oxygen over here = 6
Total formal positive charge on Sulfur = +6
Oxidation number of sulfur= +6
As oxidation number of sulfur is its highest possible oxidation number and no oxygen-oxygen bond is present over here so the name of this ion will be Sulfate.
Persulfate ion: ${{S}_{2}}O_{8}^{2-}$
Sulfite ion: $SO_{3}^{2-}$
Hyposulfite ion: ${{S}_{2}}O_{3}^{2-}$
Sulfide ion: ${{S}^{2-}}$
Additional Information : Sulfates occur as microscopic particles (aerosols) resulting from fossil and biomass combustion. They increase the acidity of the atmosphere and form acid rain.
Note: Sulfate ion present in general along with phosphate for qualitative analysis of ions. Confirmatory tests of sulfate ion are as follows:
a.) Aqueous solution or sodium carbonate extract of the salt acidified with acetic acid in addition to barium chloride gives a white precipitate of barium sulfate insoluble in conc. $HCl$ or conc. $HN{{O}_{3}}$ .
b.) Sulfate ions give white precipitate of lead sulphate when aqueous solution or sodium carbonate extract neutralised with acetic acid is treated with lead acetate solution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




