
What is the name of the compound \[Al{I_3}\]?
A) Ammonium iodide
B) Aluminium iodide
C) Aluminium (I) iodide
D) None of the above
Answer
524.7k+ views
Hint: We have to remember that before solving this question you must know the electronic configuration of aluminium
\[A{l^ + }\]= \[1{s^2}2{s^2}2{p^6}3{s^2}\]
\[A{l^{ + 3}}\]= \[1{s^2}2{s^2}2{p^6}\]
We must have to know that aluminium iodine is formed by the reaction of aluminium and iodine which is a combination of aluminum hydroxide with hydrochloric acid.
Complete step by step answer:
We have to remember that \[Al{I_3}\] is the compound having IUPAC Name Aluminium iodide. It can also be written as Aluminium (III) iodide.
\[Al\] has an atomic number 13 and electronic configuration of \[Al\] is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^1}\].
Aluminium iodide is a compound that contains \[Al\] and \[I\] atoms. This molecule has a trigonal planar which exhibits a dimer form which is a monomer and forms a bridged dimer \[A{l_2}{I_6}\].
Bridged dimer: \[A{l_2}{I_6}\] can be represented as
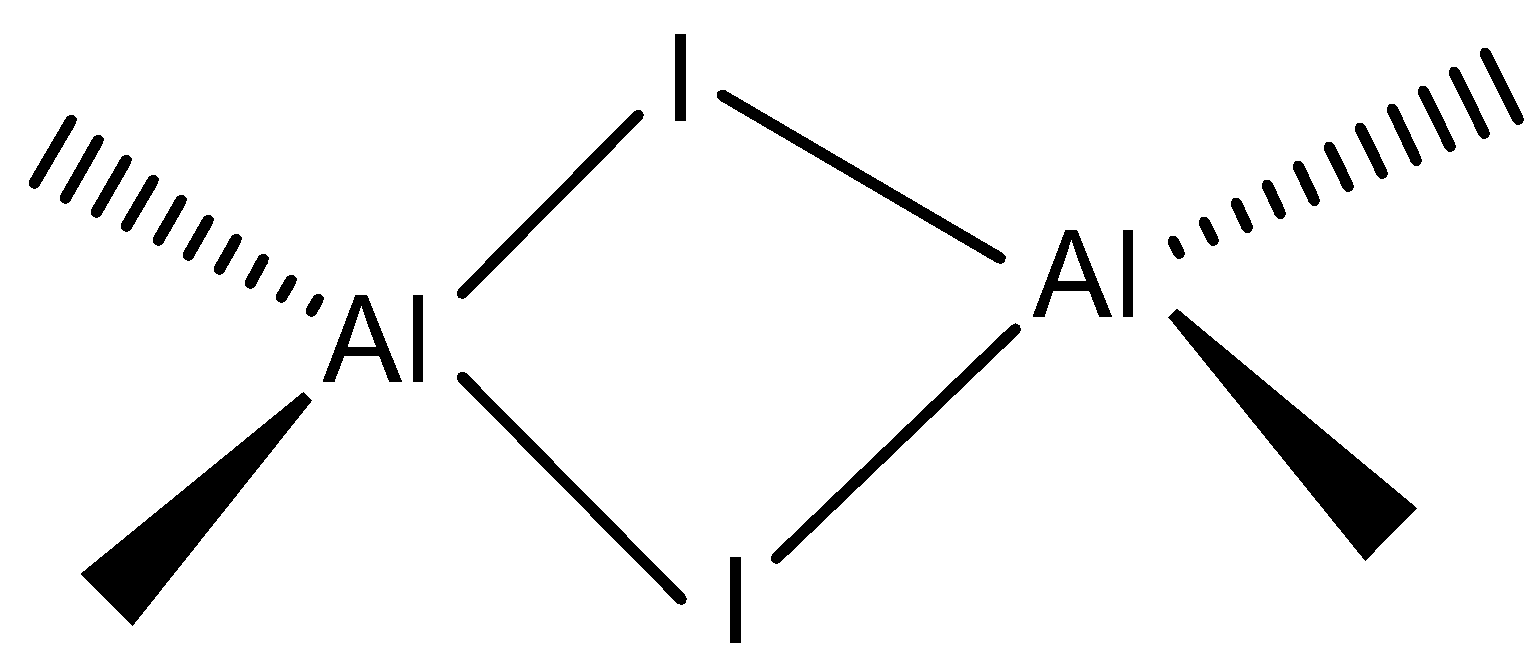
Based on the above concept let’s look at the options:
Option A) this is an incorrect option as ammonium iodide has a molecular formula as \[N{H_4}I\].
Option B) This option is correct as Aluminium iodide has a molecular formula \[Al{I_3}\].
\[Al\] on losing $3$ electrons will become \[A{l^{ + 3}}\], therefore valency or charge on \[Al\] is \[ + 3\] due to which while cross balancing it will become Aluminium (III) iodide which is also an IUPAC Name of Aluminium iodide.
Option C) this is an incorrect option, as this option represents \[Al\] in \[ + 1\] oxidation state which is not asked in the question. Al on losing an electron will become \[A{l^ + }\] therefore the formula turns out to be \[AlI\].
Option D) this is an incorrect option as we got the correct option as option B.
Hence, the correct answer is, ‘Option B’.
Note: We have to remember that the aluminium iodide is used to illustrate \[Al{I_3}\] as a dimer. We must remember that the \[Al{I_3}\] is a monomeric unit to form a dimer which has a molecular formula \[A{l_2}{I_6}\].
\[A{l^ + }\]= \[1{s^2}2{s^2}2{p^6}3{s^2}\]
\[A{l^{ + 3}}\]= \[1{s^2}2{s^2}2{p^6}\]
We must have to know that aluminium iodine is formed by the reaction of aluminium and iodine which is a combination of aluminum hydroxide with hydrochloric acid.
Complete step by step answer:
We have to remember that \[Al{I_3}\] is the compound having IUPAC Name Aluminium iodide. It can also be written as Aluminium (III) iodide.
\[Al\] has an atomic number 13 and electronic configuration of \[Al\] is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^1}\].
Aluminium iodide is a compound that contains \[Al\] and \[I\] atoms. This molecule has a trigonal planar which exhibits a dimer form which is a monomer and forms a bridged dimer \[A{l_2}{I_6}\].
Bridged dimer: \[A{l_2}{I_6}\] can be represented as

Based on the above concept let’s look at the options:
Option A) this is an incorrect option as ammonium iodide has a molecular formula as \[N{H_4}I\].
Option B) This option is correct as Aluminium iodide has a molecular formula \[Al{I_3}\].
\[Al\] on losing $3$ electrons will become \[A{l^{ + 3}}\], therefore valency or charge on \[Al\] is \[ + 3\] due to which while cross balancing it will become Aluminium (III) iodide which is also an IUPAC Name of Aluminium iodide.
Option C) this is an incorrect option, as this option represents \[Al\] in \[ + 1\] oxidation state which is not asked in the question. Al on losing an electron will become \[A{l^ + }\] therefore the formula turns out to be \[AlI\].
Option D) this is an incorrect option as we got the correct option as option B.
Hence, the correct answer is, ‘Option B’.
Note: We have to remember that the aluminium iodide is used to illustrate \[Al{I_3}\] as a dimer. We must remember that the \[Al{I_3}\] is a monomeric unit to form a dimer which has a molecular formula \[A{l_2}{I_6}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




