
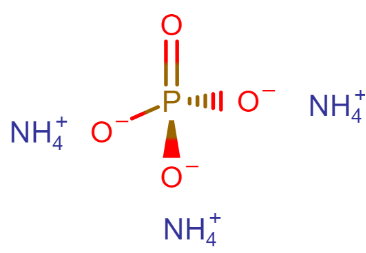
What is the name of the compound $ {(N{H_4})_3}P{O_4} $ ?
Answer
506.4k+ views
Hint : $ {(N{H_4})_3}P{O_4} $ is an unstable inorganic compound made up of ammonium and phosphate salt. Its molecular mass is $ 149.09{g \mathord{\left/
{\vphantom {g {mol}}} \right.
} {mol}} $ and density $ 1.619{g \mathord{\left/
{\vphantom {g {cubi{c_{}}cm}}} \right.
} {cubi{c_{}}cm}} $ , chemical formula $ {H_9}{N_2}{O_4}P $ .
Complete Step By Step Answer:
The name of the compound Is Ammonium phosphate, other names- triammonium phosphate, diazonium hydrogen phosphate, are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
Triammonium phosphate can be prepared in the laboratory by treating $ 85\% $ phosphoric acid with $ 30\% $ ammonia solution:
$ {H_3}P{O_4} + 3N{H_3} \to {(N{H_4})_3}P{O_4} $
$ {(N{H_4})_3}P{O_4} $ is a colorless, crystalline solid. The solid, which has the odor of ammonia, is readily soluble in water. The salt converts to diammonium hydrogen phosphate $ {(N{H_4})_3}HP{O_4} $ .
Chemical properties:
$ \bullet $ Ammonium phosphate readily undergoes decomposition reaction emitting very toxic fumes. It forms phosphoric acid and ammonia.
$ {(N{H_4})_3}P{O_4} \to 3N{H_3} + {H_3}P{O_4} $
$ \bullet $ Ammonium phosphate reacts with lead nitrate forming lead phosphate and ammonium nitrate.
$ 4{(N{H_4})_3}P{O_4} + 3Pb{(N{O_3})_4} \to P{b_3}{(P{O_4})_4} + 12N{H_4}N{O_3} $
Ammonium phosphate is soluble in water, and ammonia loses and the acid phosphate $ (N{H_4}) $ $ ({H_2}P{O_4}) $ is formed as the aqueous solution on the boil. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers, this is also used in thermoplastic formulations as a flame retardant.
Uses:
Ammonium phosphate is a broad generic name for a variety of fertilizers containing both nitrogen and phosphate. Used as components of intumescent paints and mastics where they function as an acid catalyst. Used in paints.
Note :
Ammonium phosphate is also manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transforms the ammonium phosphate sulphate to the molten state. It includes a group of nitrogen phosphorus materials, mono ammonium phosphates, mixtures of the two or combination with ammonium nitrate or ammonium sulfate.
{\vphantom {g {mol}}} \right.
} {mol}} $ and density $ 1.619{g \mathord{\left/
{\vphantom {g {cubi{c_{}}cm}}} \right.
} {cubi{c_{}}cm}} $ , chemical formula $ {H_9}{N_2}{O_4}P $ .

Complete Step By Step Answer:
The name of the compound Is Ammonium phosphate, other names- triammonium phosphate, diazonium hydrogen phosphate, are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
Triammonium phosphate can be prepared in the laboratory by treating $ 85\% $ phosphoric acid with $ 30\% $ ammonia solution:
$ {H_3}P{O_4} + 3N{H_3} \to {(N{H_4})_3}P{O_4} $
$ {(N{H_4})_3}P{O_4} $ is a colorless, crystalline solid. The solid, which has the odor of ammonia, is readily soluble in water. The salt converts to diammonium hydrogen phosphate $ {(N{H_4})_3}HP{O_4} $ .
Chemical properties:
$ \bullet $ Ammonium phosphate readily undergoes decomposition reaction emitting very toxic fumes. It forms phosphoric acid and ammonia.
$ {(N{H_4})_3}P{O_4} \to 3N{H_3} + {H_3}P{O_4} $
$ \bullet $ Ammonium phosphate reacts with lead nitrate forming lead phosphate and ammonium nitrate.
$ 4{(N{H_4})_3}P{O_4} + 3Pb{(N{O_3})_4} \to P{b_3}{(P{O_4})_4} + 12N{H_4}N{O_3} $
Ammonium phosphate is soluble in water, and ammonia loses and the acid phosphate $ (N{H_4}) $ $ ({H_2}P{O_4}) $ is formed as the aqueous solution on the boil. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers, this is also used in thermoplastic formulations as a flame retardant.
Uses:
Ammonium phosphate is a broad generic name for a variety of fertilizers containing both nitrogen and phosphate. Used as components of intumescent paints and mastics where they function as an acid catalyst. Used in paints.
Note :
Ammonium phosphate is also manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transforms the ammonium phosphate sulphate to the molten state. It includes a group of nitrogen phosphorus materials, mono ammonium phosphates, mixtures of the two or combination with ammonium nitrate or ammonium sulfate.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




