
How do you name the alkynes with two triple bonds?
Answer
527.4k+ views
Hint: Alkynes are basically hydrocarbon compounds in which the carbon atom containing a triple bond is $ s{{p}^{2}} $ hybridized. Alkynes are a class of organic compounds and we know that for nomenclature or naming of any organic compounds we need to follow the rules specified by the IUPAC.
Complete answer:
So, we will first study the nomenclature of chains with one triple bond and the rules for chains with two triple bonds are similar but the only care we need to take is the numbering and usage of ‘’bi’’, ‘’tri’’ prefixes.
Start by finding the longest carbon chain which includes both carbons of all the triple bonds.
Number the longest chain by starting from the end which is closest to the triple bond. Always remember that $ 1-alkyne~ $ is termed as a terminal alkyne whereas alkynes at any other positions are termed as internal alkynes.
Next step after numbering the longest chain with the least number assigned to the alkynes, we have to label each substituent at its corresponding carbon.
While writing the name of the molecules, always arrange the substituents in alphabetical order. We should always remember that if there are multiple same substituents present then we should use the prefixes di, tri for two, three substituents respectively. These prefixes are not written as per the alphabetical order rule. For example:
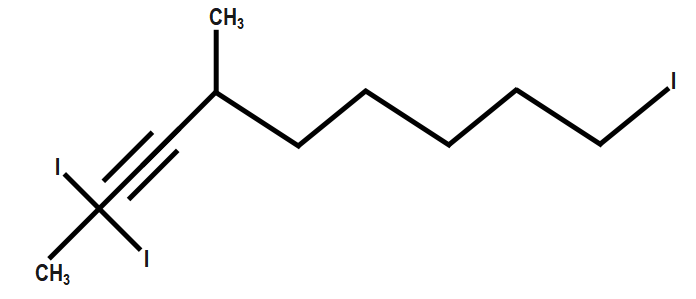
$ 1-triiodo-4-dimethyl-2-nonyne $
Always remember that if there is an alcohol present in a molecule, then number the longest chain by starting at the end closest to alcohol and follow similar rules. However, take care that the suffix in this case would be –ynol, as the alcohol group has more priority than the triple bond.
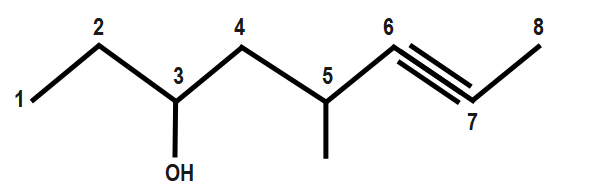 $ 5-~methyl-7-octyn-3-ol $
$ 5-~methyl-7-octyn-3-ol $
When there are two triple bonds in the molecule, then also the steps are as similar to the first four rules. We have to find the longest carbon chain which includes both the triple bonds and we should take care that we have to number the longest chain starting from the end which is closest to the first triple bond. The suffix that will be used for naming of this molecule will be –di-yne.
Let us understand with the help of the following figure.
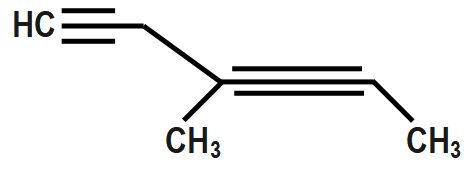
Here we will start the numbering from the left-hand side with the carbon containing the first alkyne group, getting number one and the other alkyne group getting number $ 3. $ while doing so, we have assigned the least number to both the triple bonds.
Note:
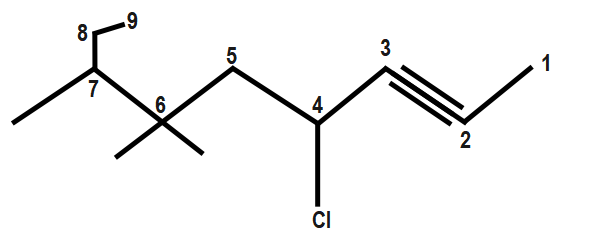
Always remember that sometimes double and triple bonds both are present in a compound that means a compound contains both the double and triple bonds. In such compounds always prefer double bond over the triple bond and thus numbering should be done such that double bond or alkene gets the least number.
Complete answer:
So, we will first study the nomenclature of chains with one triple bond and the rules for chains with two triple bonds are similar but the only care we need to take is the numbering and usage of ‘’bi’’, ‘’tri’’ prefixes.
Start by finding the longest carbon chain which includes both carbons of all the triple bonds.
Number the longest chain by starting from the end which is closest to the triple bond. Always remember that $ 1-alkyne~ $ is termed as a terminal alkyne whereas alkynes at any other positions are termed as internal alkynes.
For example:

Next step after numbering the longest chain with the least number assigned to the alkynes, we have to label each substituent at its corresponding carbon.
While writing the name of the molecules, always arrange the substituents in alphabetical order. We should always remember that if there are multiple same substituents present then we should use the prefixes di, tri for two, three substituents respectively. These prefixes are not written as per the alphabetical order rule. For example:

$ 1-triiodo-4-dimethyl-2-nonyne $
Always remember that if there is an alcohol present in a molecule, then number the longest chain by starting at the end closest to alcohol and follow similar rules. However, take care that the suffix in this case would be –ynol, as the alcohol group has more priority than the triple bond.

When there are two triple bonds in the molecule, then also the steps are as similar to the first four rules. We have to find the longest carbon chain which includes both the triple bonds and we should take care that we have to number the longest chain starting from the end which is closest to the first triple bond. The suffix that will be used for naming of this molecule will be –di-yne.
Let us understand with the help of the following figure.

Here we will start the numbering from the left-hand side with the carbon containing the first alkyne group, getting number one and the other alkyne group getting number $ 3. $ while doing so, we have assigned the least number to both the triple bonds.
Note:
Always remember that sometimes double and triple bonds both are present in a compound that means a compound contains both the double and triple bonds. In such compounds always prefer double bond over the triple bond and thus numbering should be done such that double bond or alkene gets the least number.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




