
Name the functional group for which cannizzaro reaction is not given?
Answer
522.6k+ views
Hint: Cannizzaro reaction is a reduction – oxidation reaction, in short we can say, it is a redox reaction. To solve this question first we need to study about the criteria for the reaction to take place and later on the mechanism of the cannizzaro reaction.
Complete answer:
As we know, Cannizzaro is a redox reaction. In this reaction two molecules of aldehyde undergo disproportionation reaction i.e. one molecule of aldehyde gets oxidized and converts to carboxylic acid while the other molecule undergoes reduction and converts to alcohol.
And the criteria for this reaction to happen is that the molecule should not have\[\alpha - H\]. Now to be clear \[\alpha - H\] is the atom attached to \[\alpha - C\]of the molecule. And the \[\alpha - C\]of the molecule is the C just adjacent to the C bearing functional group.
So, we can say those molecules or aldehydes that will have \[\alpha - H\] will not give a Cannizzaro reaction.
Example of such aldehyde is Acetaldehyde - \[C{H_3} - CHO\] , since this molecule is having \[\alpha - H\] bearing \[\alpha - C\] , so it will not undergo cannizzaro reaction.
Now let’s have a quick look at the mechanism of the cannizzaro reaction and why molecules containing \[\alpha - H\] will not undergo this reaction.
Cannizzaro reaction – e.g. – formaldehyde
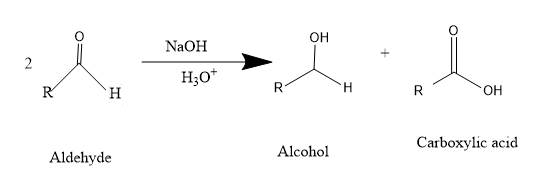
So, this reaction takes place by the nucleophilic attack of hydroxyl group \[( - OH)\] on the aldehyde carbonyl carbon \[(C = O)\] and the resulting intermediate release the much less preferable hydride anion and will attack other aldehyde and thus reaction proceeds as shown in figure.
Note:
Molecules having \[\alpha - H\] do not undergo a cannizzaro reaction because it is a less favourable reaction. It only occurs when no other option is available. Molecules with \[\alpha - H\] also undergo condensation.
Complete answer:
As we know, Cannizzaro is a redox reaction. In this reaction two molecules of aldehyde undergo disproportionation reaction i.e. one molecule of aldehyde gets oxidized and converts to carboxylic acid while the other molecule undergoes reduction and converts to alcohol.
And the criteria for this reaction to happen is that the molecule should not have\[\alpha - H\]. Now to be clear \[\alpha - H\] is the atom attached to \[\alpha - C\]of the molecule. And the \[\alpha - C\]of the molecule is the C just adjacent to the C bearing functional group.
So, we can say those molecules or aldehydes that will have \[\alpha - H\] will not give a Cannizzaro reaction.
Example of such aldehyde is Acetaldehyde - \[C{H_3} - CHO\] , since this molecule is having \[\alpha - H\] bearing \[\alpha - C\] , so it will not undergo cannizzaro reaction.
Now let’s have a quick look at the mechanism of the cannizzaro reaction and why molecules containing \[\alpha - H\] will not undergo this reaction.
Cannizzaro reaction – e.g. – formaldehyde

So, this reaction takes place by the nucleophilic attack of hydroxyl group \[( - OH)\] on the aldehyde carbonyl carbon \[(C = O)\] and the resulting intermediate release the much less preferable hydride anion and will attack other aldehyde and thus reaction proceeds as shown in figure.
Note:
Molecules having \[\alpha - H\] do not undergo a cannizzaro reaction because it is a less favourable reaction. It only occurs when no other option is available. Molecules with \[\alpha - H\] also undergo condensation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




