
Name the method you shall use to separate two immiscible liquids.
Answer
592.5k+ views
Hint: “Immiscible liquids are those which won't mix to give a single phase. Oil and water are examples of immiscible liquids. One floats on top of the other”.
Complete step by step answer:
We know that the property of immiscibility is going to depend on the polarity of the liquids.
-If two liquids are polar in nature they will miscible in one another very easily.
-If two liquids are non-polar then also they will miscible very easily.
-If one liquid is polar and the other liquid is non-polar they won’t miscible very easily and then they are called immiscible liquids.
-But it is not that much easy to separate the two immiscible liquids.
-There is a separate technique to separate those immiscible liquids.
The name of the instrument which is used to separate two immiscible liquids is called as separating funnel.
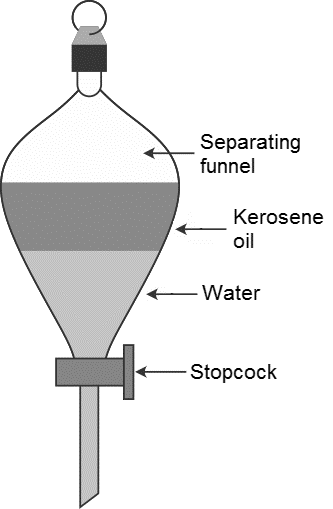
-By using the separating funnel we can easily separate two immiscible liquids.
-We have to take the two immiscible liquids into the separating funnel and close the separating funnel with a knob and leave it for a few minutes till the two layers separated.
-In the picture itself it shows that the kerosene and water are two immiscible liquids separated.
-The kerosene floats on the surface of water.
-After separation of two immiscible layers remove the knob and collect the layers separately by using a stopcock..
-First by using the stopcock we can collect the water and then kerosene in two separate beakers.
Note:
Don’t be confused with the words polar and non-polar.
Polar means the chemicals which are soluble in water are called polar.
Non-polar means the chemicals which are not soluble in water are called as non-polar.
Complete step by step answer:
We know that the property of immiscibility is going to depend on the polarity of the liquids.
-If two liquids are polar in nature they will miscible in one another very easily.
-If two liquids are non-polar then also they will miscible very easily.
-If one liquid is polar and the other liquid is non-polar they won’t miscible very easily and then they are called immiscible liquids.
-But it is not that much easy to separate the two immiscible liquids.
-There is a separate technique to separate those immiscible liquids.
The name of the instrument which is used to separate two immiscible liquids is called as separating funnel.

-By using the separating funnel we can easily separate two immiscible liquids.
-We have to take the two immiscible liquids into the separating funnel and close the separating funnel with a knob and leave it for a few minutes till the two layers separated.
-In the picture itself it shows that the kerosene and water are two immiscible liquids separated.
-The kerosene floats on the surface of water.
-After separation of two immiscible layers remove the knob and collect the layers separately by using a stopcock..
-First by using the stopcock we can collect the water and then kerosene in two separate beakers.
Note:
Don’t be confused with the words polar and non-polar.
Polar means the chemicals which are soluble in water are called polar.
Non-polar means the chemicals which are not soluble in water are called as non-polar.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




