
Name the scientist who developed the pH scale.
Answer
564.3k+ views
Hint:We know that the degree of alkalinity or acidity of a solution is known as its pH. pH is the negative logarithm of the hydrogen ion concentration. pH of a solution is determined using the pH scale
Complete solution:
We know that a pH scale is used to determine the acidic or basic nature of the substance. The pH scale ranges from 0 to 14.
If the pH of the solution is less than 7 then the solution has acidic nature. If the pH of the solution is more than 7 then the solution has basic nature. If the pH of the solution is equal to 7 then the solution is neither acidic nor basic it is neutral in nature.
Sometimes pH value can be less than 0. This indicates that the solution is highly acidic. Also sometimes the pH value can be more than 14. This indicates that the solution is highly alkaline.
pH is defined as the negative logarithm of the hydrogen ion concentration. The equation to calculate pH is as follows:
${\text{pH}} = - \log [{{\text{H}}^ + }]$
The scientist who developed pH scale is Sorenson. The pH scale is as follows:
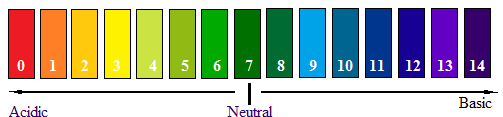
The various colours are observed at the pH value on pH paper.
Thus, the scientist who developed pH scale is Sorenson.
Note: pH scale is used to rank the solutions in terms of basicity or acidity. Other ways to measure pH are pH paper, indicators, etc. Lemon juice is acidic in nature. Sodium hydroxide is basic in nature. Distilled water is neutral in nature.
Complete solution:
We know that a pH scale is used to determine the acidic or basic nature of the substance. The pH scale ranges from 0 to 14.
If the pH of the solution is less than 7 then the solution has acidic nature. If the pH of the solution is more than 7 then the solution has basic nature. If the pH of the solution is equal to 7 then the solution is neither acidic nor basic it is neutral in nature.
Sometimes pH value can be less than 0. This indicates that the solution is highly acidic. Also sometimes the pH value can be more than 14. This indicates that the solution is highly alkaline.
pH is defined as the negative logarithm of the hydrogen ion concentration. The equation to calculate pH is as follows:
${\text{pH}} = - \log [{{\text{H}}^ + }]$
The scientist who developed pH scale is Sorenson. The pH scale is as follows:

The various colours are observed at the pH value on pH paper.
Thus, the scientist who developed pH scale is Sorenson.
Note: pH scale is used to rank the solutions in terms of basicity or acidity. Other ways to measure pH are pH paper, indicators, etc. Lemon juice is acidic in nature. Sodium hydroxide is basic in nature. Distilled water is neutral in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




