
Name the series of hydrogen spectrum which lies in the ultraviolet region.
Answer
590.1k+ views
Hint: Each element has a characteristic spectrum of radiation, which it emits. A particular element would exhibit some regular pattern with emitted light frequencies. Hydrogen is the simplest atom and therefore, has the simplest spectrum.
Complete step by step answer:
The atomic hydrogen emits a line spectrum consisting of various series. In the hydrogen spectrum, the spacing between lines within certain sets of the hydrogen spectrum decreases in regular ways. Each of these sets are called spectral series. Balmer, Lyman, Paschen, brackett, and Pfund series are subsequently found in spectral series of the hydrogen spectrum at different wavelengths.
Lyman series:
$\dfrac{1}{\lambda }=R[\dfrac{1}{{{1}^{2}}}-\dfrac{1}{{{n}^{2}}}],where,n=2,3,4...$
The Lyman series is in the ultraviolet region.
According to third postulate of Bohr’s model, when an atom makes a transition from the higher energy state with quantum number \[{{n}_{i}}\] to the lower energy state with quantum number ${{n}_{f}}({{n}_{f}}<{{n}_{i}})$ , the difference of energy is carried away by a photon of frequency ${{v}_{if}}$ such that
\[h{{v}_{if}}={{E}_{{{n}_{i}}}}-{{E}_{{{n}_{f}}}}\]
Since both ${{n}_{f}}$ and ${{n}_{i}}$ are integers, this immediately shows that in transitions between different atomic levels, light is radiated in various discrete frequencies. For hydrogen spectrum, the results of the Bohr’s model suggested the presence of other series spectra for hydrogen atom those corresponding to transitions resulting from ${{n}_{f}}=1$ and ${{n}_{i}}=2,3,etc.$ ; ${{n}_{f}}=3\And {{n}_{i}}=4,5,etc.$ and so on. Such series were identified in the course of spectroscopic investigations and are known as the Lyman, Balmer, Paschen, Brackett, and Pfund series. the electronic transitions corresponding to these series shown in the below diagram,
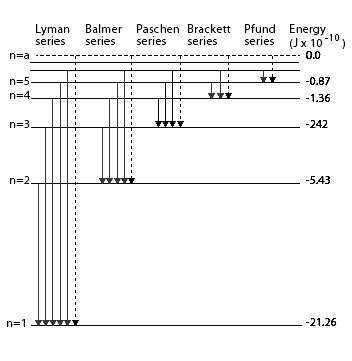
Hence, from the above hydrogen spectrum, the series which lies in the ultraviolet region is the Lyman series.
Note: The various lines in the atomic spectra are produced when electrons jump from higher energy state to lower energy state and photons are emitted. These spectral lines are called emission lines. But when an atom absorbs a photon that has precisely the same energy needed by the electron in a lower energy state to make transitions to a higher energy state, this process is called absorption.
Complete step by step answer:
The atomic hydrogen emits a line spectrum consisting of various series. In the hydrogen spectrum, the spacing between lines within certain sets of the hydrogen spectrum decreases in regular ways. Each of these sets are called spectral series. Balmer, Lyman, Paschen, brackett, and Pfund series are subsequently found in spectral series of the hydrogen spectrum at different wavelengths.
Lyman series:
$\dfrac{1}{\lambda }=R[\dfrac{1}{{{1}^{2}}}-\dfrac{1}{{{n}^{2}}}],where,n=2,3,4...$
The Lyman series is in the ultraviolet region.
According to third postulate of Bohr’s model, when an atom makes a transition from the higher energy state with quantum number \[{{n}_{i}}\] to the lower energy state with quantum number ${{n}_{f}}({{n}_{f}}<{{n}_{i}})$ , the difference of energy is carried away by a photon of frequency ${{v}_{if}}$ such that
\[h{{v}_{if}}={{E}_{{{n}_{i}}}}-{{E}_{{{n}_{f}}}}\]
Since both ${{n}_{f}}$ and ${{n}_{i}}$ are integers, this immediately shows that in transitions between different atomic levels, light is radiated in various discrete frequencies. For hydrogen spectrum, the results of the Bohr’s model suggested the presence of other series spectra for hydrogen atom those corresponding to transitions resulting from ${{n}_{f}}=1$ and ${{n}_{i}}=2,3,etc.$ ; ${{n}_{f}}=3\And {{n}_{i}}=4,5,etc.$ and so on. Such series were identified in the course of spectroscopic investigations and are known as the Lyman, Balmer, Paschen, Brackett, and Pfund series. the electronic transitions corresponding to these series shown in the below diagram,

Hence, from the above hydrogen spectrum, the series which lies in the ultraviolet region is the Lyman series.
Note: The various lines in the atomic spectra are produced when electrons jump from higher energy state to lower energy state and photons are emitted. These spectral lines are called emission lines. But when an atom absorbs a photon that has precisely the same energy needed by the electron in a lower energy state to make transitions to a higher energy state, this process is called absorption.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




