
Name two synthetic indicators.
Answer
597.3k+ views
Hint: Synthetic Indicator - An indicator prepared from artificial substances is known as a synthetic indicator. In simple words, all the indicators which are not natural are known as man-made or synthetic indicators.
Complete step-by-step answer:
> The two synthetic indicators are phenolphthalein and methyl orange.
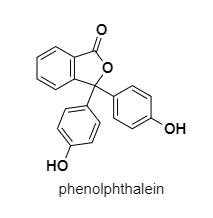
1) Phenolphthalein is an indicator used to determine the pH of the various solutions. Its solution is prepared by dissolving it in alcohol. Its solution is colorless. When a few drops of phenolphthalein are added to a basic solution it changes into a deep pink color. It remains colorless in acidic and neutral solutions.
> Phenolphthalein is used as an indicator of pH change when the pH is between ${ 8.2 }$ and ${ 10 }$ (basic solutions).
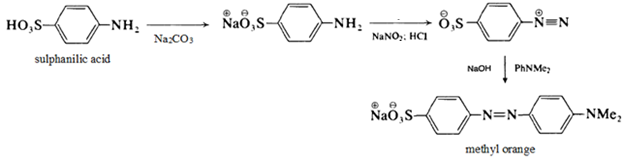
2) Methyl orange is a pH indicator frequently used in titrations.
It is often used in titrations because of its clear and distinct color change. Since it changes color at the pH of a mid-strength acid, it is normally utilized in titrations for acids. Unlike a universal indicator, methyl orange does not have a full spectrum of color change but has a sharper endpoint.
> Methyl orange is used as an indicator of pH change when the pH is between ${ 3.1 }$ and ${ 4.4 }$ (acidic solutions).
Note: The possibility to make a mistake is that in the pH range where both the indicators change the colors. In phenolphthalein, it is colorless but becomes pink above 8.2 pH and the color change interval of methyl orange is 3.1 to 4.5 pH range.
Complete step-by-step answer:
> The two synthetic indicators are phenolphthalein and methyl orange.
1) Phenolphthalein is an indicator used to determine the pH of the various solutions. Its solution is prepared by dissolving it in alcohol. Its solution is colorless. When a few drops of phenolphthalein are added to a basic solution it changes into a deep pink color. It remains colorless in acidic and neutral solutions.
> Phenolphthalein is used as an indicator of pH change when the pH is between ${ 8.2 }$ and ${ 10 }$ (basic solutions).

2) Methyl orange is a pH indicator frequently used in titrations.
It is often used in titrations because of its clear and distinct color change. Since it changes color at the pH of a mid-strength acid, it is normally utilized in titrations for acids. Unlike a universal indicator, methyl orange does not have a full spectrum of color change but has a sharper endpoint.
> Methyl orange is used as an indicator of pH change when the pH is between ${ 3.1 }$ and ${ 4.4 }$ (acidic solutions).

Note: The possibility to make a mistake is that in the pH range where both the indicators change the colors. In phenolphthalein, it is colorless but becomes pink above 8.2 pH and the color change interval of methyl orange is 3.1 to 4.5 pH range.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




