
Natural rubber or raw rubber consists of basic material latex which is a dispersion of isoprene. During the treatment, this isoprene forms a high molecular weight polymer of isoprene. Natural rubber can be obtained from five hundred different species of plants.
In the isoprene polymer all the isoprene units have which of the following:
A.Trans 1, 4 configuration
B.Cis 1, 4 configuration
C.Both cis and trans 1, 4 configuration
D.None of these
Answer
590.1k+ views
Hint: Natural rubber is an additional polymer of a substance which is an unsaturated hydrocarbon obtained generally in processing petroleum or coal tar.
Complete step by step answer:
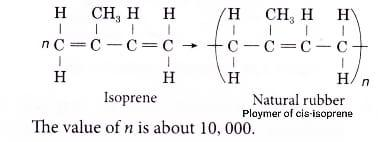
Natural rubber is the cis-isomer of poly-isoprene and is formed from isoprene.Its IUPAC name is 2-methyl-1,3-butadiene.
The monomer of natural rubber is Isoprene. Poly-isoprene is a polymer of isoprene $({{C}_{5}}{{H}_{8}})$ that is the primary chemical constituent of natural rubber. Many properties of rubber make it a very useful compound like Specific gravity, Abrasion resistance, Tear resistance, Compression set, Resilience, Elongation, Tensile modulus, Tensile strength.
Hence, the correct answer is cis-2-methyl-1,3-butadiene or isoprene which is option B.
Note:
To convert raw rubber into tough and useful product vulcanization of rubber is done. Sulphur is added to polymer and heated. This makes sulphur atoms cross link the adjacent polymer chains.
This cross linking improves following properties of rubber:
-A tougher material that is more resistant to oxidation
-More elastic material as cross linked chains can revert back to their positions
-It becomes more heat resistant
-And less soluble in organic solvents.
Complete step by step answer:
Natural rubber is the cis-isomer of poly-isoprene and is formed from isoprene.Its IUPAC name is 2-methyl-1,3-butadiene.
The monomer of natural rubber is Isoprene. Poly-isoprene is a polymer of isoprene $({{C}_{5}}{{H}_{8}})$ that is the primary chemical constituent of natural rubber. Many properties of rubber make it a very useful compound like Specific gravity, Abrasion resistance, Tear resistance, Compression set, Resilience, Elongation, Tensile modulus, Tensile strength.
Hence, the correct answer is cis-2-methyl-1,3-butadiene or isoprene which is option B.

Note:
To convert raw rubber into tough and useful product vulcanization of rubber is done. Sulphur is added to polymer and heated. This makes sulphur atoms cross link the adjacent polymer chains.
This cross linking improves following properties of rubber:
-A tougher material that is more resistant to oxidation
-More elastic material as cross linked chains can revert back to their positions
-It becomes more heat resistant
-And less soluble in organic solvents.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




