
$NiC{{l}_{2}}$ in the presence of dimethyl glyoxime (DMG) gives a complex which precipitates in the presence of $N{{H}_{4}}OH$, giving a bright red colour. Predict whether it is diamagnetic or paramagnetic.
(A) paramagnetic
(B) diamagnetic
(C) both
(D) none
Answer
591.9k+ views
Hint: Write the reaction which takes place in this process. DMG is a bidentate ligand so calculate the number of electrons donated by it to the central metal atom. Write the electronic configuration of nickel and identify the type of hybridization it undergoes in the complex formed.
Complete step by step answer:
-First, let us start by writing the reaction,
$NiC{{l}_{2}}+dmg\xrightarrow{N{{H}_{4}}OH}\left[ Ni{{\left( dmg \right)}_{2}} \right]+N{{H}_{4}}Cl+{{H}_{2}}O$
-Nickel chloride reacts with dimethylglyoxime in the presence of ammonium hydroxide to form a bright red coloured precipitate of bis-(dimethylglyoximato)nickel(II) complex. The structure of the complex is represented as \[Ni-{{\left( dmg \right)}_{2}}\] and shown below.
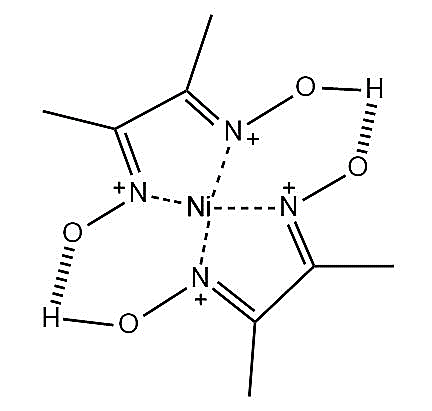
-Let’s write the electronic configuration of nickel. Nickel has atomic number 28.
\[Ni=\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{8}}=\left[ Ar \right]3{{d}^{8}}4{{s}^{2}}\]
-The structure of dimethylglyoxime is as follows:
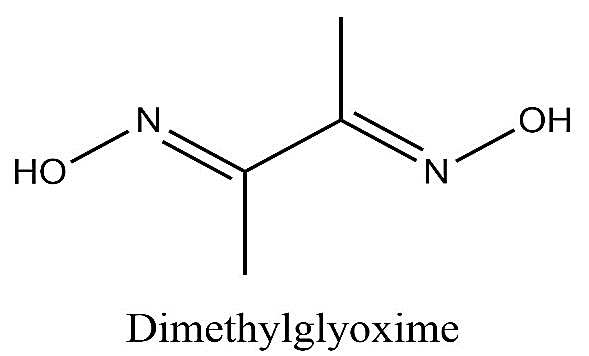
-DMG is a bidentate ligand. So, it has two donor nitrogen atoms which means one dmg donates four electrons to the nickel atom. So, two dmg molecules will donate eight electrons to form the complex.
-Nickel exists in +2 oxidation state in the complex. So, the electronic configuration will be $\left[ Ar \right]3{{d}^{8}}4{{s}^{0}}$.
-Nickel in its +2-oxidation state, has vacant 4s and 4p orbitals. Ni undergoes $ds{{p}^{2}}$hybridization to form a complex with dmg.
-DMG is a low spin ligand so all the electrons filled in the $ds{{p}^{2}}$ will be paired. There will not be any unpaired electrons present in the complex.
-Due to absence of unpaired electrons, \[Ni-{{\left( dmg \right)}_{2}}\] complex will be diamagnetic in nature.
So, the correct answer is “Option B”.
Note: Remember if there is presence of unpaired electrons then the complex is paramagnetic and if no unpaired electrons are present then, the complex is diamagnetic. Always consider the spectrochemical series while filling electrons in the orbitals of metal. If the ligand is high spin then the arrangement will be different.
Complete step by step answer:
-First, let us start by writing the reaction,
$NiC{{l}_{2}}+dmg\xrightarrow{N{{H}_{4}}OH}\left[ Ni{{\left( dmg \right)}_{2}} \right]+N{{H}_{4}}Cl+{{H}_{2}}O$
-Nickel chloride reacts with dimethylglyoxime in the presence of ammonium hydroxide to form a bright red coloured precipitate of bis-(dimethylglyoximato)nickel(II) complex. The structure of the complex is represented as \[Ni-{{\left( dmg \right)}_{2}}\] and shown below.

-Let’s write the electronic configuration of nickel. Nickel has atomic number 28.
\[Ni=\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{8}}=\left[ Ar \right]3{{d}^{8}}4{{s}^{2}}\]
-The structure of dimethylglyoxime is as follows:
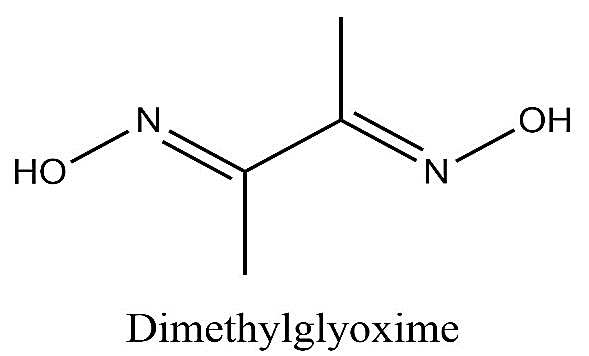
-DMG is a bidentate ligand. So, it has two donor nitrogen atoms which means one dmg donates four electrons to the nickel atom. So, two dmg molecules will donate eight electrons to form the complex.
-Nickel exists in +2 oxidation state in the complex. So, the electronic configuration will be $\left[ Ar \right]3{{d}^{8}}4{{s}^{0}}$.
-Nickel in its +2-oxidation state, has vacant 4s and 4p orbitals. Ni undergoes $ds{{p}^{2}}$hybridization to form a complex with dmg.
-DMG is a low spin ligand so all the electrons filled in the $ds{{p}^{2}}$ will be paired. There will not be any unpaired electrons present in the complex.
-Due to absence of unpaired electrons, \[Ni-{{\left( dmg \right)}_{2}}\] complex will be diamagnetic in nature.
So, the correct answer is “Option B”.
Note: Remember if there is presence of unpaired electrons then the complex is paramagnetic and if no unpaired electrons are present then, the complex is diamagnetic. Always consider the spectrochemical series while filling electrons in the orbitals of metal. If the ligand is high spin then the arrangement will be different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




