
Nitration of aniline in the strong acidic medium also gives $m - $ nitroaniline because ________.
A: In absence of substitutes, the nitro group always goes to $m - $ positions.
B: In spite of substituents, the nitro group always goes to only $m - $ positions.
C: In strong acidic medium aniline is present as anilinium ion.
D: In electrophilic substitution reactions, the amino group is Meta directive.
Answer
592.5k+ views
Hint: $m - $ nitroaniline is also called $3 - $ nitroaniline. It is a volatile substance used as a raw material in production of dyes. It is stable in neutral, basic as well as acidic medium. It is classified as not readily biodegradable.
Complete step by step answer:
In nitration of aniline nitric acid oxidizes aniline to anilinium ion. This anilinium ion is a meta directive due to which $m - $ nitroaniline is formed. ${C_6}{H_5}NH_3^ + $ is anilinium ion. In this nitrogen is already having four bonds (one with benzene ring and three with other hydrogen atoms) this means it has no lone pair. This means there will be no conjugation (shifting of negative charge) but there is positive charge on nitrogen atom this means there will be negative inductive effect due to nitrogen. Due to which position where positive charge will be farthest from nitrogen atom will be most stable also these farthest positions are para and then Meta. Due to which Meta nitroaniline is also formed.
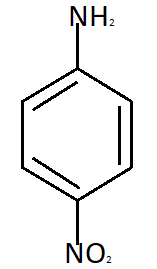
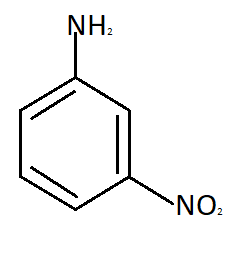
Para nitroaniline meta nitroaniline
So the reason due to which Meta nitroaniline is formed is formation of anilinium ion which imposes the inductive effect. So the correct answer is option C that is in strong acidic medium aniline is present as anilinium ion.
Note:
When an electron donating group is attached to an alkyl group then the charge is relayed through the chain it is called positive inductive effect. Similarly when an electron withdrawing group is attached negative inductive effect will take place.
Complete step by step answer:
In nitration of aniline nitric acid oxidizes aniline to anilinium ion. This anilinium ion is a meta directive due to which $m - $ nitroaniline is formed. ${C_6}{H_5}NH_3^ + $ is anilinium ion. In this nitrogen is already having four bonds (one with benzene ring and three with other hydrogen atoms) this means it has no lone pair. This means there will be no conjugation (shifting of negative charge) but there is positive charge on nitrogen atom this means there will be negative inductive effect due to nitrogen. Due to which position where positive charge will be farthest from nitrogen atom will be most stable also these farthest positions are para and then Meta. Due to which Meta nitroaniline is also formed.


Para nitroaniline meta nitroaniline
So the reason due to which Meta nitroaniline is formed is formation of anilinium ion which imposes the inductive effect. So the correct answer is option C that is in strong acidic medium aniline is present as anilinium ion.
Note:
When an electron donating group is attached to an alkyl group then the charge is relayed through the chain it is called positive inductive effect. Similarly when an electron withdrawing group is attached negative inductive effect will take place.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




