
Nitration of phenyl benzoate yields the product:
A)
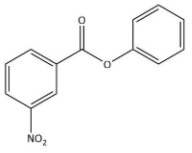
B)
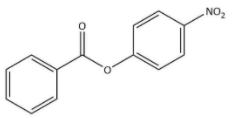
C)
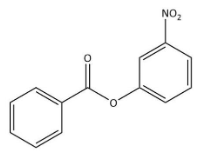
D)
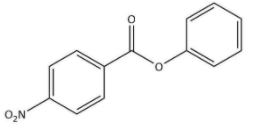




Answer
585.6k+ views
Hint: We know that the presence of electron withdrawing groups such as ${NO}_{2}$, ${CN}$ etc. at o-and p-positions but not at m-positions with respect to the halogen greatly activates the halogen towards nucleophilic displacement. We also know that the number of such groups at o- and p-positions with respect to the halogens varies directly with the reactivity of the haloarene.
Complete answer:
We know that the ring which is attached to the O- atom, in that the nitro group enters and as the result of it $\text C_{6}\text H_{5}\text {COO}-$ group gets activated. It is known that it will not get into the ring to which the carbonyl group as it will deactivate the $\text C_{6}\text H_{5}\text {COO}-$ group. We can now say that the $\text C_{6}\text H_{5}\text {COO}-$ group is ortho and para directing in nature.
The chemical equation that represents the formation of 4-nitrophenyl benzoate is shown as follows.
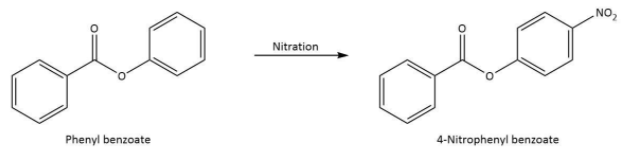
Thus, we can conclude the nitration of phenyl benzoate yields 4-nitrophenyl benzoate.
Hence, we can conclude that the correct option is B.
Note: We know that the presence of electron withdrawing groups at o- and p-position but not at m-positions with respect to the halogens activates the aryl halides towards nucleophilic substitution reactions. The number of nitro groups at o- and p-positions increases, the stabilization of the resulting carbanion increases due to more resonating structures which results in the greater reactivity of the corresponding aryl halide.
Complete answer:
We know that the ring which is attached to the O- atom, in that the nitro group enters and as the result of it $\text C_{6}\text H_{5}\text {COO}-$ group gets activated. It is known that it will not get into the ring to which the carbonyl group as it will deactivate the $\text C_{6}\text H_{5}\text {COO}-$ group. We can now say that the $\text C_{6}\text H_{5}\text {COO}-$ group is ortho and para directing in nature.
The chemical equation that represents the formation of 4-nitrophenyl benzoate is shown as follows.

Thus, we can conclude the nitration of phenyl benzoate yields 4-nitrophenyl benzoate.
Hence, we can conclude that the correct option is B.
Note: We know that the presence of electron withdrawing groups at o- and p-position but not at m-positions with respect to the halogens activates the aryl halides towards nucleophilic substitution reactions. The number of nitro groups at o- and p-positions increases, the stabilization of the resulting carbanion increases due to more resonating structures which results in the greater reactivity of the corresponding aryl halide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




