
Nitrous acid ($HN{{O}_{2}}$) converts amino acids into hydroxy acids with retention of configuration. Estimation of nitrogen gas evolved in the reaction is the basis of Van slyke estimation of amino acids.
\[R-CH(N{{H}_{2}})-COOH\xrightarrow{HN{{O}_{2}}}R-CH(OH)-COOH+{{N}_{2}}\uparrow +{{H}_{2}}O\]
Which of the following amino acids cannot be analyzed by Van slyke method?
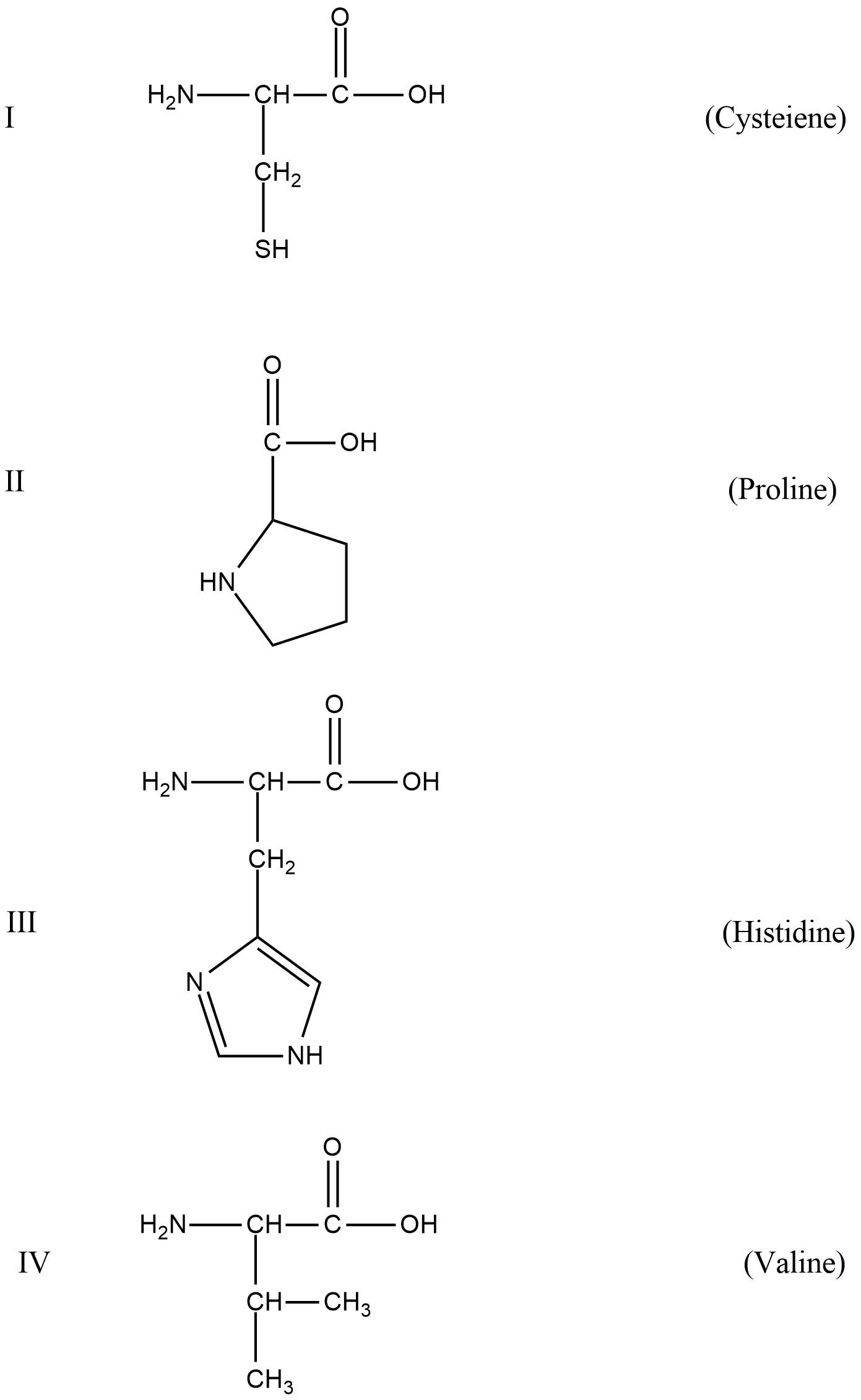
A. Only I
B. Only II
C. I and III
D. I, III, IV

Answer
515.4k+ views
Hint: Van slyke method is useful to determine the presence of the primary amine functional group in the amino acid. In van slyke method the primary amines are going to be converted to hydroxides with the help of the nitrous acid.
Complete answer:
- In the question it is asked to find which amino acid among the given amino acids is not going to participate in van slyke method.
- The given amino acids in the question are Cysteine, proline, histidine and valine.
- Now we have to check which amino acids are not going to contain a primary amine group in their structure.
- The first amino acid is cysteine.
- The structure of cysteine is as follows.
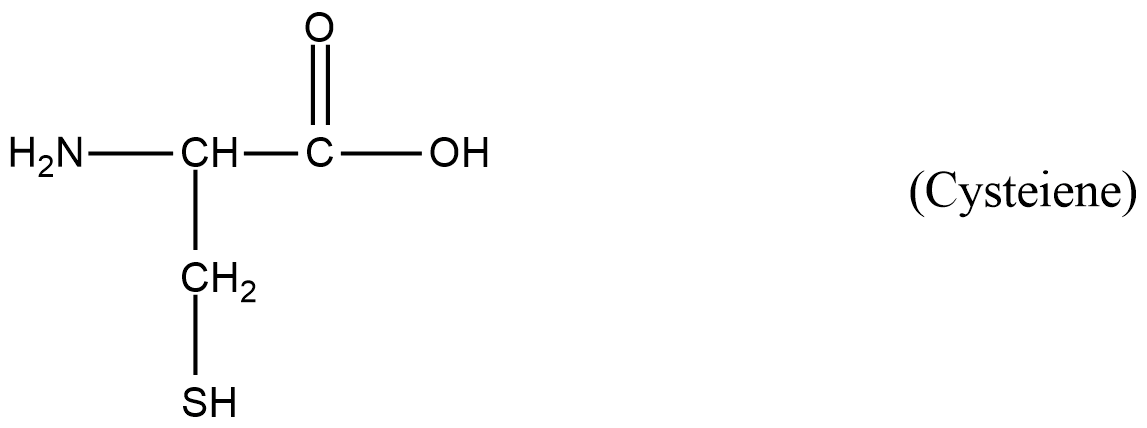
- In the above structure of amino acid cysteine, we can see that there is a primary amine. So, cysteine is going to respond to van slyke method.
- Coming to the second given amino acid.
- The structure of proline is as follows.
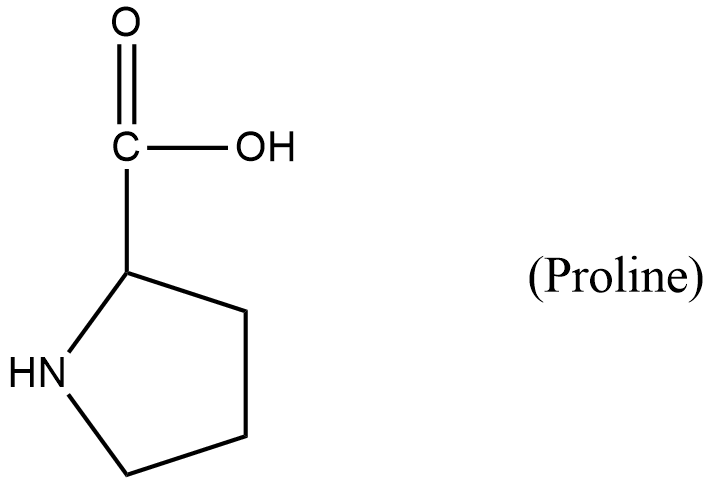
- In proline the amine is a secondary amine and that is present in the ring structure. So, the amino acid proline is not going to participate in the reaction with nitrous acid.
- So, proline does not undergo van slyke method.
- The third amino acid is Histidine.
- The structure of histidine is as follows.
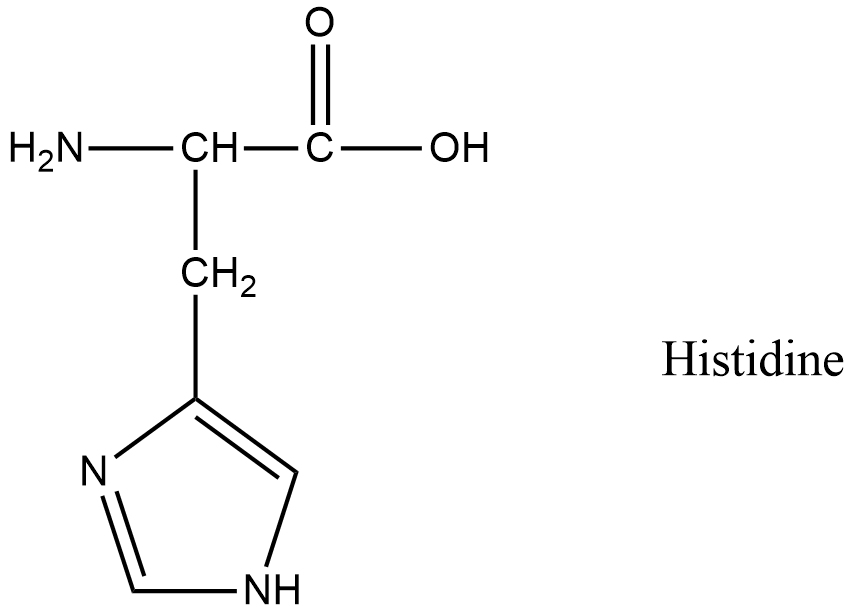
- In histidine there is a primary amine.
- So, histidine undergoes van slyke method.
- Coming to the fourth amino acid valine.
- The structure of valine is as follows.
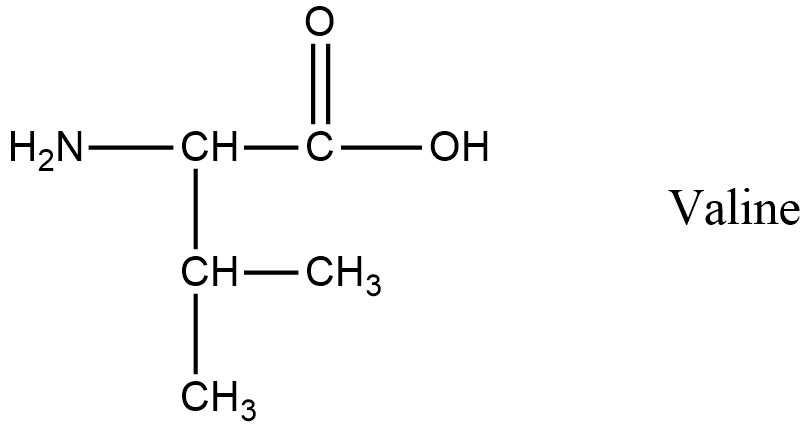
- In Valine there is a primary amine.
- So, valine undergoes van slyke method.
Therefore, the correct option is B.
Note:
The amino acids which have a primary amine in their structure only undergo van slyke method. The amino acids which do not contain a primary amine in their structure like proline does not like with nitrous acid means it is not involved in van slyke method.
Complete answer:
- In the question it is asked to find which amino acid among the given amino acids is not going to participate in van slyke method.
- The given amino acids in the question are Cysteine, proline, histidine and valine.
- Now we have to check which amino acids are not going to contain a primary amine group in their structure.
- The first amino acid is cysteine.
- The structure of cysteine is as follows.

- In the above structure of amino acid cysteine, we can see that there is a primary amine. So, cysteine is going to respond to van slyke method.
- Coming to the second given amino acid.
- The structure of proline is as follows.

- In proline the amine is a secondary amine and that is present in the ring structure. So, the amino acid proline is not going to participate in the reaction with nitrous acid.
- So, proline does not undergo van slyke method.
- The third amino acid is Histidine.
- The structure of histidine is as follows.

- In histidine there is a primary amine.
- So, histidine undergoes van slyke method.
- Coming to the fourth amino acid valine.
- The structure of valine is as follows.

- In Valine there is a primary amine.
- So, valine undergoes van slyke method.
Therefore, the correct option is B.
Note:
The amino acids which have a primary amine in their structure only undergo van slyke method. The amino acids which do not contain a primary amine in their structure like proline does not like with nitrous acid means it is not involved in van slyke method.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




