
What is the number of donor atoms in dimethylglyoximato ligand ?
(A) 1
(B) 2
(C) 3
(D) 4
Answer
593.1k+ views
Hint: There are more than one donor atoms in dimethylglyoximato. Nitrogen has a lone pair of electrons while oxygen in dimethylglyoximato is involved in hydrogen bonding. It is also called bidentate ligand.
Complete step by step answer:
To find the number of donor atoms in dimethylglyoximato, first we can give a close observation on the structure of dimethylglyoximato.
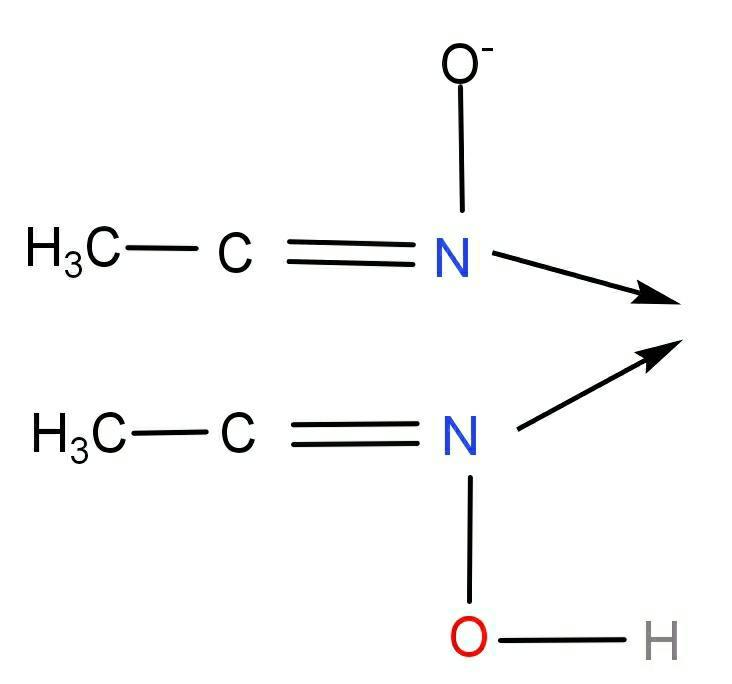
In this compound, Both the Nitrogen atoms are donors. O is involved in hydrogen bonding and because of that reason Oxygen atoms cannot be a donor.
So, it is clear that there are two donor atoms (N and N) in dimethylglyoximato.
Therefore the number of donor atoms in dimethylglyoximato is 2.
Option (B) 2 is the correct answer.
Additional Information:
Dimethyl glyoximato is also known as ‘dmg’.
Density of a ligand is defined as the number of lone pairs of electrons that can be donated by a ligand to the central metal ion.
In the case of dimethylglyoximato, its density is two, (Bidentate ligand). Bidentate ligands are of two types : symmetric and asymmetric. Symmetric ligands are those that have both the donor atoms same whereas asymmetric ligands are those having two different donor atoms.
In the case of dmg, we know that the two donor atoms are the same (N and N). and therefore dimethylglyoximato is an example of asymmetric ligand. Charge of dimethylglyoximato is -1 and so it is an anionic ligand.
Dimethylglyoxime (DMG) is used as an analytical reagent used to precipitate Ni from its aqueous solution. Here Nickel complexes with dmg form octahedral, square planar and tetrahedral complexes in +2 oxidation state. The hybridization of Ni in the complex , $\left[ Ni{ \left( DMG \right) }_{ 2 } \right] $ is ${ dsp }^{ 2 }$.
The following reaction gives the complexation of Ni with dmg.
Chelation and intramolecular hydrogen bonding are the two main factors which provide stability to this compound. Oxidation state of Ni in $\left[ Ni{ \left( DMG \right) }_{ 2 } \right] $ is +2 and has a square planar geometry because of chelation the pairing of electrons takes place. As all electrons are paired so this complex is diamagnetic and nickel with coordination number four.
Note: In dmg, even though oxygen carries a lone pair of electrons along with it, it cannot be a donor here, since the lone pair of oxygen is involved in hydrogen bonding.
Complete step by step answer:
To find the number of donor atoms in dimethylglyoximato, first we can give a close observation on the structure of dimethylglyoximato.

In this compound, Both the Nitrogen atoms are donors. O is involved in hydrogen bonding and because of that reason Oxygen atoms cannot be a donor.
So, it is clear that there are two donor atoms (N and N) in dimethylglyoximato.
Therefore the number of donor atoms in dimethylglyoximato is 2.
Option (B) 2 is the correct answer.
Additional Information:
Dimethyl glyoximato is also known as ‘dmg’.
Density of a ligand is defined as the number of lone pairs of electrons that can be donated by a ligand to the central metal ion.
In the case of dimethylglyoximato, its density is two, (Bidentate ligand). Bidentate ligands are of two types : symmetric and asymmetric. Symmetric ligands are those that have both the donor atoms same whereas asymmetric ligands are those having two different donor atoms.
In the case of dmg, we know that the two donor atoms are the same (N and N). and therefore dimethylglyoximato is an example of asymmetric ligand. Charge of dimethylglyoximato is -1 and so it is an anionic ligand.
Dimethylglyoxime (DMG) is used as an analytical reagent used to precipitate Ni from its aqueous solution. Here Nickel complexes with dmg form octahedral, square planar and tetrahedral complexes in +2 oxidation state. The hybridization of Ni in the complex , $\left[ Ni{ \left( DMG \right) }_{ 2 } \right] $ is ${ dsp }^{ 2 }$.
The following reaction gives the complexation of Ni with dmg.
${ Ni }^{ 2+ }\ +\ { 2DMG\ \left( dimethylglyoxime \right) \ \rightarrow \ \left[ Ni{ \left( DMG \right) }_{ 2 } \right] }\downarrow \ \left( bright\ red \right) $
Chelation and intramolecular hydrogen bonding are the two main factors which provide stability to this compound. Oxidation state of Ni in $\left[ Ni{ \left( DMG \right) }_{ 2 } \right] $ is +2 and has a square planar geometry because of chelation the pairing of electrons takes place. As all electrons are paired so this complex is diamagnetic and nickel with coordination number four.
Note: In dmg, even though oxygen carries a lone pair of electrons along with it, it cannot be a donor here, since the lone pair of oxygen is involved in hydrogen bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




