
Number of hydrogen-bonded water molecules associated in \[CuS{O_4}.5{H_2}O\] is:
A.One
B.Two
C.Three
D.All the five
Answer
584.7k+ views
Hint: We have to draw a structure of copper sulphate (\[CuS{O_4}.5{H_2}O\]) and then only we can check out for coordinated bonds and hydrogen bonds presents in copper sulphate.
Complete step by step solution:
As we can see in the below structure, there are a total five water molecules in the structure of copper sulphate. Out of these 5 molecules of water present in the crystal framework of copper sulphate molecule, four water molecules are coordinated to\[C{u^{ + 2}}\], while one is hydrogen bonded with \[S{O_4}^{2 - }\]
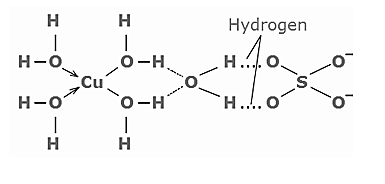
Copper sulfate pentahydrate contains copper (II) in a geometry of distorted octahedral.
As we can see in structure, the copper is bonded to four water molecules in a square-planar geometry and two oxygen atoms from two sulfate ions. In another way, we can say one \[{H_2}O\]molecule is H-Bonded here with sulphate ions.
So, 4 water molecules are in coordinated with \[C{u^{2 + }}\]ion while the 5th water molecule is hydrogen bonded with oxygen of sulphate ion. Finally, the fifth water molecule is hydrogen-bonded and is deeply embedded in a crystal and this is not coordinated.
Hence, we can conclude that only 4 water molecules are coordinated and the fifth is the only hydrogen bonded.
Hence, option A is correct.
Note: We must know that when one atom shares a pair of electrons with another atom lacking such a pair then the bond formation that occurs in them is a covalent chemical bond. It is also known as coordinated bonds.
After drawing the structure of a given compound or molecule, one can basically check if the shared pair of electrons are coming from the same molecule or not. If it is coming from the same molecule, then the compound contains a coordinate covalent bond.
Complete step by step solution:
As we can see in the below structure, there are a total five water molecules in the structure of copper sulphate. Out of these 5 molecules of water present in the crystal framework of copper sulphate molecule, four water molecules are coordinated to\[C{u^{ + 2}}\], while one is hydrogen bonded with \[S{O_4}^{2 - }\]

Copper sulfate pentahydrate contains copper (II) in a geometry of distorted octahedral.
As we can see in structure, the copper is bonded to four water molecules in a square-planar geometry and two oxygen atoms from two sulfate ions. In another way, we can say one \[{H_2}O\]molecule is H-Bonded here with sulphate ions.
So, 4 water molecules are in coordinated with \[C{u^{2 + }}\]ion while the 5th water molecule is hydrogen bonded with oxygen of sulphate ion. Finally, the fifth water molecule is hydrogen-bonded and is deeply embedded in a crystal and this is not coordinated.
Hence, we can conclude that only 4 water molecules are coordinated and the fifth is the only hydrogen bonded.
Hence, option A is correct.
Note: We must know that when one atom shares a pair of electrons with another atom lacking such a pair then the bond formation that occurs in them is a covalent chemical bond. It is also known as coordinated bonds.
After drawing the structure of a given compound or molecule, one can basically check if the shared pair of electrons are coming from the same molecule or not. If it is coming from the same molecule, then the compound contains a coordinate covalent bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




