
Number of possible isomers for \[{C_2}{H_2}Cl\] are
A. 0
B. 1
C. 2
D. 3
E. 4
F. 5
G. 6
H. 7
I. 8
J. 9
Answer
535.8k+ views
Hint: Isomerism is a very important phenomenon in organic chemistry. It is said that compounds having similar molecule formulas can have different structural formulas and this phenomenon is known as isomerism.
Complete step-by-step answer:
Structural isomerism is divided into three parts.
Chain isomerism: If compounds contain similar molecular formula, but they differ in the formation of chain, that is the parent chain of each of the compounds is different than they are called chain isomerism.
Position isomerism: If the compound contains similar molecular formula but they differ in the position of the main functional group of the unsaturated bonds, then this type of compounds are considered into position isomerism.
Functional group isomerism: If the compound contains similar molecular formula but they differ in the presence of the functional group, that is the functional group present on one compound is aldehyde but the functional group present on the other compound is ketone or alcohol.
Now in the question, we are provided with \[{C_2}{H_2}Cl\]. Since in the molecular formula we have only two carbon atoms, the presence of chain isomerism is not possible. Also, the formula only consists of hydrogen and chlorine atoms, that is, there is the only formation of alkyl halide possible, and therefore, functional isomerism is also not possible.
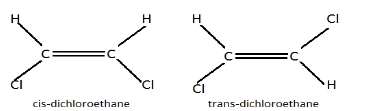
One is the cis isomer, cause both the halogen group is present on one side of the compound, the second one is the trans isomer, cause both the halogen group is placed on the opposite side of each other.
Thus, the correct answer for the given question is option C.
Note: There is two Isomer formed out of the above compound that is cis and trans. Cis isomer is less stable than the trans isomer because, since the functional group is present on the same side it produces hindrance. On the other hand, in trans-isomers, both the functional group is present on the opposite sides of each other and forms a balance.
Complete step-by-step answer:
Structural isomerism is divided into three parts.
Chain isomerism: If compounds contain similar molecular formula, but they differ in the formation of chain, that is the parent chain of each of the compounds is different than they are called chain isomerism.
Position isomerism: If the compound contains similar molecular formula but they differ in the position of the main functional group of the unsaturated bonds, then this type of compounds are considered into position isomerism.
Functional group isomerism: If the compound contains similar molecular formula but they differ in the presence of the functional group, that is the functional group present on one compound is aldehyde but the functional group present on the other compound is ketone or alcohol.
Now in the question, we are provided with \[{C_2}{H_2}Cl\]. Since in the molecular formula we have only two carbon atoms, the presence of chain isomerism is not possible. Also, the formula only consists of hydrogen and chlorine atoms, that is, there is the only formation of alkyl halide possible, and therefore, functional isomerism is also not possible.

One is the cis isomer, cause both the halogen group is present on one side of the compound, the second one is the trans isomer, cause both the halogen group is placed on the opposite side of each other.
Thus, the correct answer for the given question is option C.
Note: There is two Isomer formed out of the above compound that is cis and trans. Cis isomer is less stable than the trans isomer because, since the functional group is present on the same side it produces hindrance. On the other hand, in trans-isomers, both the functional group is present on the opposite sides of each other and forms a balance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




