
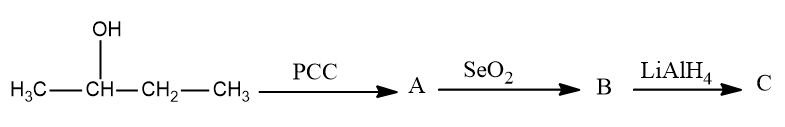
What is the Number of possible stereoisomers in C
(A) $ 2 $
(B) $ 3. $
(C) $ 4 $
(D) $ 5 $

Answer
540.9k+ views
Hint :We know that isomers are the molecules which have the same molecular formula but different structural formula. Mainly isomers are divided into two types- the structural isomers and the geometrical or stereoisomers. So, stereoisomers are the ones which have the same composition (or same parts) but those parts differ when oriented in space. Further stereoisomers are divided into diastereomers and enantiomers.
Complete Step By Step Answer:
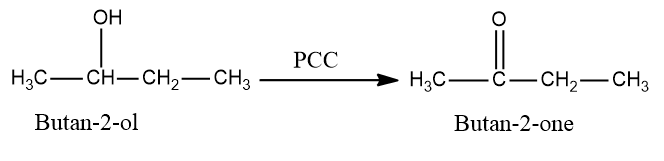
In the first step we will convert $ {\text{butan}} - 2 - ol $ into $ {\text{butan}} - 2 - one $ as $ PCC $ is an oxidizing agent which converts alcohols into aldehydes or ketones but is not strong enough to further convert them into carboxylic acid so the reaction will stop when an aldehyde or a ketone is formed.
So, the conversion equation will be: -
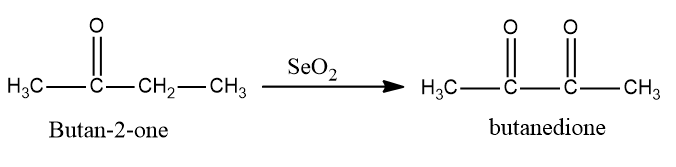
Now in the next step selenium dioxide is used to convert $ {\text{butan}} - 2 - one $ into butanedione as $ Se{O_2} $ is also an oxidizing agent which converts alkenes into alcohols and further converts alcohol into aldehydes or ketones.
So, the conversion equation will be: -
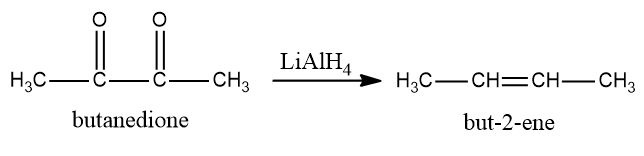
Now we see that butanedione will be converted into but $ {\text{butan}} - 2 - ene $ is a reducing agent and is being added in the last step. $ LiAl{H_4} $ is a reducing agent and will first convert ketone into alcohol and then into alkene.
So, the conversion equation will be: -
Therefore, in the given equation: -
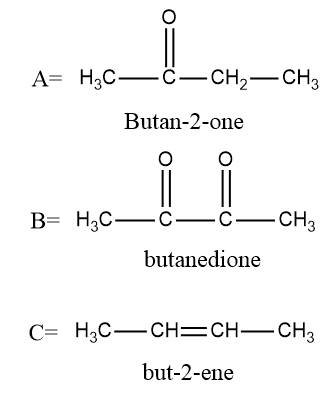
To determine the number of stereoisomers possible in $ {\text{butan}} - 2 - ene $ we will draw the structures
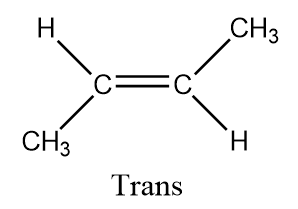
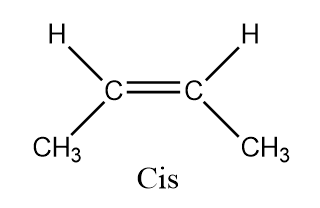
Hence there are two types of ismores possible in this and hence it forms $ {\text{Cis - butan}} - 2 - ene $ and $ {\text{Trans - butan}} - 2 - ene $
So, the correct answer is Option A.
Note :
Here $ {\text{butan}} - 2 - ene $ is a diastereomer. Diastereomers are stereoisomers which are not the mirror images of one another and are not superimposable on one another. Also, cis isomers are the isomers those have the two single hydrogen atoms present on the same side while trans have the two single hydrogen atoms present on the opposite sides.
Complete Step By Step Answer:
In the first step we will convert $ {\text{butan}} - 2 - ol $ into $ {\text{butan}} - 2 - one $ as $ PCC $ is an oxidizing agent which converts alcohols into aldehydes or ketones but is not strong enough to further convert them into carboxylic acid so the reaction will stop when an aldehyde or a ketone is formed.
So, the conversion equation will be: -

Now in the next step selenium dioxide is used to convert $ {\text{butan}} - 2 - one $ into butanedione as $ Se{O_2} $ is also an oxidizing agent which converts alkenes into alcohols and further converts alcohol into aldehydes or ketones.
So, the conversion equation will be: -

Now we see that butanedione will be converted into but $ {\text{butan}} - 2 - ene $ is a reducing agent and is being added in the last step. $ LiAl{H_4} $ is a reducing agent and will first convert ketone into alcohol and then into alkene.
So, the conversion equation will be: -

Therefore, in the given equation: -

To determine the number of stereoisomers possible in $ {\text{butan}} - 2 - ene $ we will draw the structures


Hence there are two types of ismores possible in this and hence it forms $ {\text{Cis - butan}} - 2 - ene $ and $ {\text{Trans - butan}} - 2 - ene $
So, the correct answer is Option A.
Note :
Here $ {\text{butan}} - 2 - ene $ is a diastereomer. Diastereomers are stereoisomers which are not the mirror images of one another and are not superimposable on one another. Also, cis isomers are the isomers those have the two single hydrogen atoms present on the same side while trans have the two single hydrogen atoms present on the opposite sides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




