
Number of $\sigma $ and $\pi $ bonds in ${{C}_{2}}$ molecule is/are?
A. 1$\sigma $ and 1$\pi $
B. 1$\sigma $ and 2 $\pi $
C. 2$\pi $
D. 1$\sigma $ and 3 $\pi $
Answer
573.9k+ views
Hint: Valence bond theory predicts covalent bond formation between atoms when they have half-filled valence atomic orbital, each containing a single unpaired electron. For example, sigma and pi bonds may overlap. Sigma bonds form when the two shared electrons have orbits that overlap head-to-head.
Complete Solution :
Diatomic carbon (${{C}_{2}}$) is a green, gaseous inorganic chemical. It is kinetically unstable at ambient temperature and pressure, being removed through auto polymerisation. Diatomic carbon is the second simplest form of carbon after atomic carbon. It is a gas that only exists above $3,642{}^\circ C$ below which it aggregates into graphite. It occurs in carbon vapour for example in blue hydrocarbon flames.
Now let us discuss the structure of ${{C}_{2}}$-
Molecular Orbital Theory shows that there are two sets of paired electrons in a degenerate $\pi $ bonding set of orbitals. Which means the bond order is 2. So there should exist a double bond between two carbon atoms in ${{C}_{2}}$ molecule. It is estimated that carbon vapour is around 28% diatomic, but theoretically it is dependent on the temperature and pressure. Their double bonds are made of two $\pi $ bonds because four electrons need to be accommodated in each bond. In bond formation only valence electrons or outermost electrons participate.
We can show the molecular orbital diagram pictorially as-
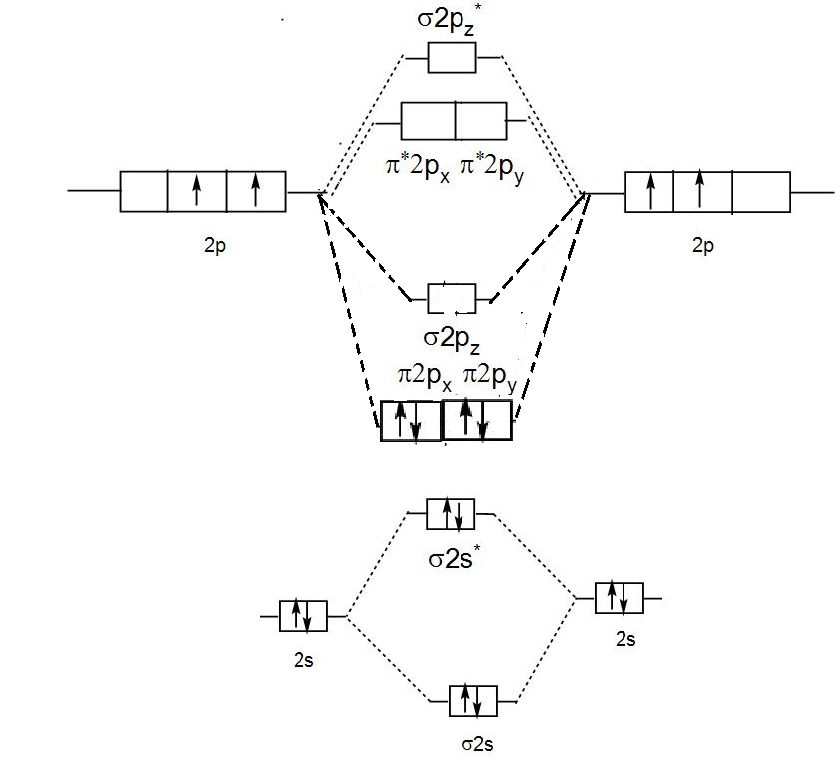
Hence, in ${{C}_{2}}$ molecules only 2$\pi $ are present.
So, the correct answer is “Option C”.
Note: We should know that sigma and pi are types of covalent bond.
Generally, there are three types of covalent bonding that we all know about. They are-
- Single bond- When there's a sharing of two electrons between a pair of atoms, it gives rise to a sigma bond between the atoms. The sigma bond thus formed is the single bond.
- Double bond- When four electrons are shared by the 2 atoms, it gives rise to a sigma bond and a pi-bond which we know as a double bond.
- Triple bond- When six electrons are shared by the two atoms, there exist one sigma and two pi-bonds thus forming a triple bond.
Complete Solution :
Diatomic carbon (${{C}_{2}}$) is a green, gaseous inorganic chemical. It is kinetically unstable at ambient temperature and pressure, being removed through auto polymerisation. Diatomic carbon is the second simplest form of carbon after atomic carbon. It is a gas that only exists above $3,642{}^\circ C$ below which it aggregates into graphite. It occurs in carbon vapour for example in blue hydrocarbon flames.
Now let us discuss the structure of ${{C}_{2}}$-
Molecular Orbital Theory shows that there are two sets of paired electrons in a degenerate $\pi $ bonding set of orbitals. Which means the bond order is 2. So there should exist a double bond between two carbon atoms in ${{C}_{2}}$ molecule. It is estimated that carbon vapour is around 28% diatomic, but theoretically it is dependent on the temperature and pressure. Their double bonds are made of two $\pi $ bonds because four electrons need to be accommodated in each bond. In bond formation only valence electrons or outermost electrons participate.
We can show the molecular orbital diagram pictorially as-

Hence, in ${{C}_{2}}$ molecules only 2$\pi $ are present.
So, the correct answer is “Option C”.
Note: We should know that sigma and pi are types of covalent bond.
Generally, there are three types of covalent bonding that we all know about. They are-
- Single bond- When there's a sharing of two electrons between a pair of atoms, it gives rise to a sigma bond between the atoms. The sigma bond thus formed is the single bond.
- Double bond- When four electrons are shared by the 2 atoms, it gives rise to a sigma bond and a pi-bond which we know as a double bond.
- Triple bond- When six electrons are shared by the two atoms, there exist one sigma and two pi-bonds thus forming a triple bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




