
Number of the possible geometrical isomers for 1,3 – pentadiene is:
A.8
B.4
C.3
D.2
Answer
561.6k+ views
Hint:1,3 – pentadiene appears as a clear colorless liquid with an acrid colour. A dangerous fire risk. Vapors are irritating to the eyes and respiratory system. Subject to polymerization if heated or contaminated. If the polymerization takes place inside a container, the container may violently rupture. Insoluble in water. Used to make intermediates and polymers.
Complete answer:
There are only two isomers possible for 1,3 – pentadine. The geometrical isomers are:
Cis
Trans
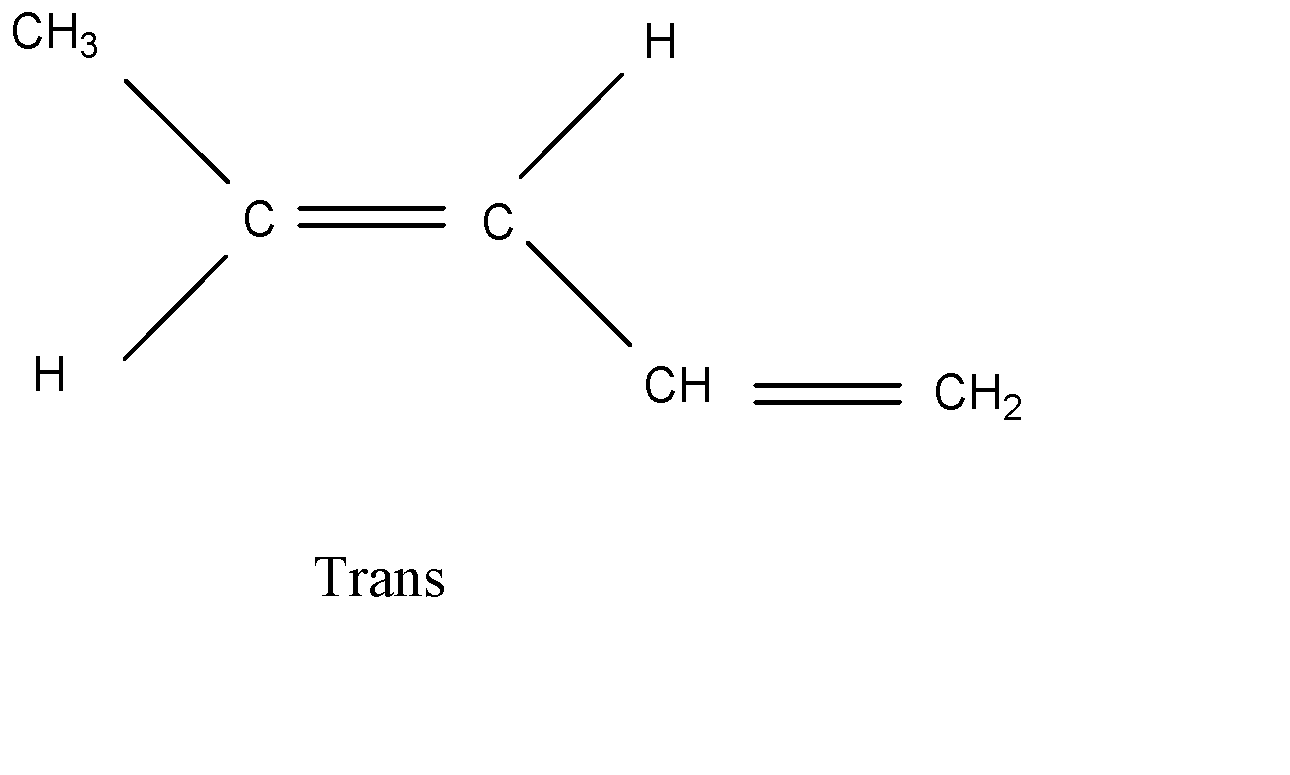
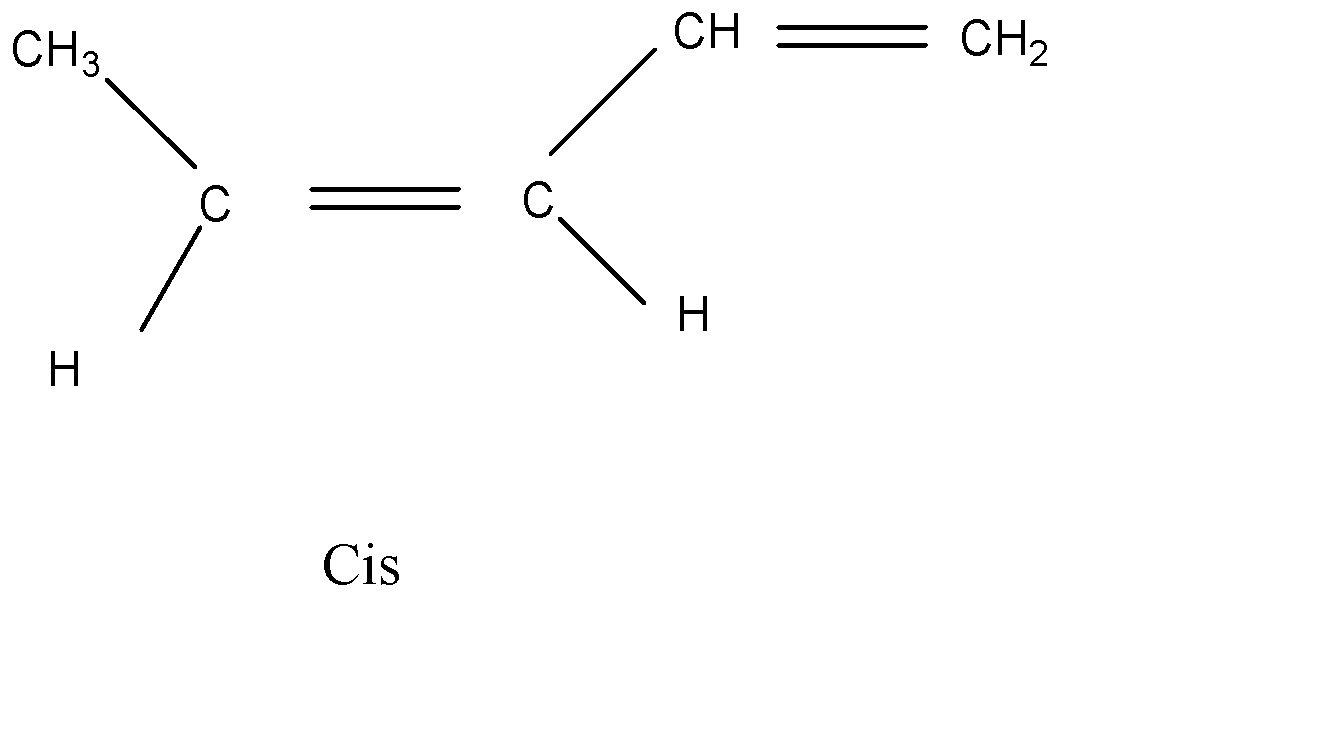
Hence, option (D) is the correct answer.
Additional information:
Molecular formula of 1,3 – pentadiene is ${C_5}{H_8}$ . Isomerism the existence of molecules that have the same numbers of the same kinds of atoms but differ in chemical and physical properties. Such structure also would be analogous to isomers. In a more suitable analogy, One’s hands can be seen as isomeric. The roots of the word isomer are Greek-iso plus meroes or equal parts. Two make crude analogy, two bracelets, each consisting of five red and five beds, could be arranged in many different isomeric forms, depending on the order of the colours.
Note:
Structural isomers are molecules which have the same molecular formula but have different connectivities. Alkanes can be very simple examples of this. Isomers are compounds that contain exactly the same number of atoms. They have exactly the same empirical formula, but differ from each other by the way in which the atoms are arranged. There can be several isomers for each empirical formula.
Complete answer:
There are only two isomers possible for 1,3 – pentadine. The geometrical isomers are:
Cis
Trans


Hence, option (D) is the correct answer.
Additional information:
Molecular formula of 1,3 – pentadiene is ${C_5}{H_8}$ . Isomerism the existence of molecules that have the same numbers of the same kinds of atoms but differ in chemical and physical properties. Such structure also would be analogous to isomers. In a more suitable analogy, One’s hands can be seen as isomeric. The roots of the word isomer are Greek-iso plus meroes or equal parts. Two make crude analogy, two bracelets, each consisting of five red and five beds, could be arranged in many different isomeric forms, depending on the order of the colours.
Note:
Structural isomers are molecules which have the same molecular formula but have different connectivities. Alkanes can be very simple examples of this. Isomers are compounds that contain exactly the same number of atoms. They have exactly the same empirical formula, but differ from each other by the way in which the atoms are arranged. There can be several isomers for each empirical formula.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




