
How to obtain phthalic acid from aspirin?
Answer
536.1k+ views
Hint:Preparation of phthalic acid from aspirin is not that much easy because the oxygen in the acetate group in the aspirin is attached to the benzene ring, not the carbon and the carboxylic group in aspirin is the meta directing group.
The chemical structures of phthalic acid and aspirin are as follows.
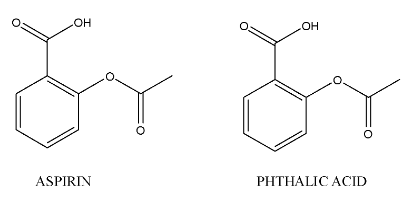
Complete step-by-step answer:- In the question it is given that to prepare phthalic acid from aspirin.
- The main chemical reaction to synthesize phthalic acid from aspirin is as follows.
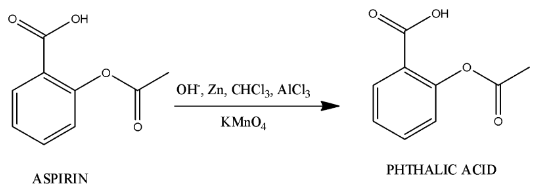
- The process of preparation of phthalic acid from aspirin is not a single step process, it is a multi-step process.
- The mechanism to prepare phthalic acid from aspirin is as follows
Step-1
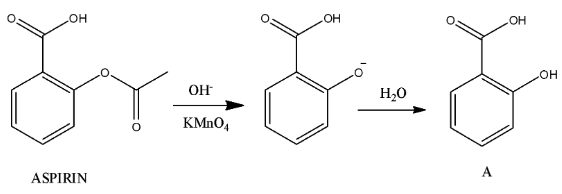
- In the above chemical reaction hydrolysis of the ester generated alcohol nothing but compound-A.
Step-2
- In the second step the formed alcohol is going to reduce by using zinc and the chemical reaction is as follows.
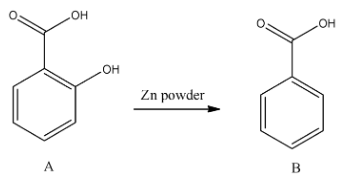
- The reduction of A gives B (benzoic acid).
Step-3
-In the third step lithium aluminium hydride is going to reduce the carboxylic acid to alcohol.
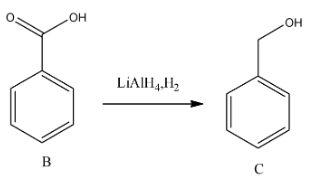
Step-4
- In the fourth step in the presence of aluminum chloride alkylation is going to do with methyl chloride.
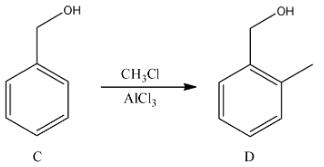
Step-5
- In the next step the product D is going to be treated with potassium permanganate to the phthalic acid compound E as the product.
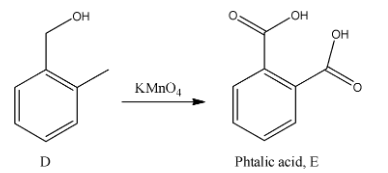
Note:It is not possible to get the phthalic acid from aspirin. It takes five steps to prepare phthalic acid from aspirin. This many steps are due to the presence of the carboxylic acid functional group in the aspirin structure.
The chemical structures of phthalic acid and aspirin are as follows.

Complete step-by-step answer:- In the question it is given that to prepare phthalic acid from aspirin.
- The main chemical reaction to synthesize phthalic acid from aspirin is as follows.

- The process of preparation of phthalic acid from aspirin is not a single step process, it is a multi-step process.
- The mechanism to prepare phthalic acid from aspirin is as follows
Step-1

- In the above chemical reaction hydrolysis of the ester generated alcohol nothing but compound-A.
Step-2
- In the second step the formed alcohol is going to reduce by using zinc and the chemical reaction is as follows.

- The reduction of A gives B (benzoic acid).
Step-3
-In the third step lithium aluminium hydride is going to reduce the carboxylic acid to alcohol.

Step-4
- In the fourth step in the presence of aluminum chloride alkylation is going to do with methyl chloride.

Step-5
- In the next step the product D is going to be treated with potassium permanganate to the phthalic acid compound E as the product.

Note:It is not possible to get the phthalic acid from aspirin. It takes five steps to prepare phthalic acid from aspirin. This many steps are due to the presence of the carboxylic acid functional group in the aspirin structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




