
What is obtained when Benzoyl Chloride reacts with Aniline in presence of Sodium Hydroxide?
(A) Benzoic acid
(B) Benzanilide
(C) Acetanilide
(D) Azobenzene
Answer
521.4k+ views
Hint: Nucleophilic N-atoms in Aniline can attack electrophilic atoms of Benzoyl Chloride and give a reaction. The Name of this reaction is Schotten-Baumann reaction.
Complete Step-by-Step Solution:
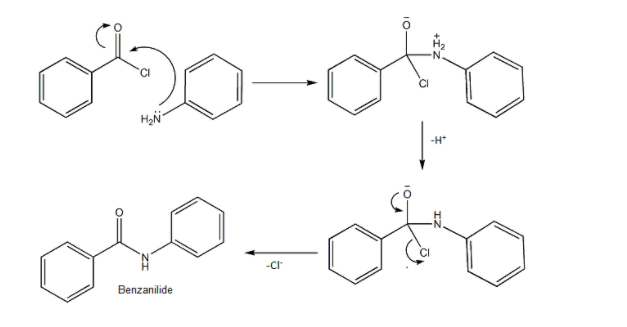
As shown in the mechanism, the N-atom of aniline is nucleophilic and it attacks the carbonyl carbon of Benzoyl chloride. Carbonyl carbon is often a preferred place for nucleophilic attack.
- Then Proton is lost and consequently, Chlorine anion is removed from the molecule to give neutral product Benzanilide.
- We can see that HCl is also formed during the reaction. So presence of Sodium hydroxide not only catalyses the reaction but also neutralizes the effect of acid formed in the reaction.
- So, option (B) Benzanilide is correct.
Additional Information:
- This reaction involves formation of a C-N bond. We can categorize this reaction to Condensation Reactions.
-A Condensation reaction is a type of a reaction in which two molecules combine to give a product and small neutral molecules like water, Ammonia etc…
- Benzanilide is used as Fungicide and Pesticide as well.
- In this Schotten-Baumann reaction, Pyridine can also be used as a catalyst in place of Sodium hydroxide.
Note:
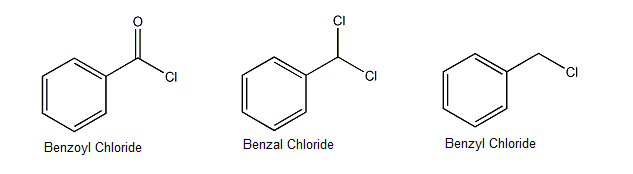
- Do not get confused between Benzoyl chloride, Benzyl Chloride, Benzal Chloride and Benzo Chloride whose structures are as shown below.
Here no suitable conditions or reagents are present for electrophilic aromatic substitution, So, do not consider it.
Complete Step-by-Step Solution:

As shown in the mechanism, the N-atom of aniline is nucleophilic and it attacks the carbonyl carbon of Benzoyl chloride. Carbonyl carbon is often a preferred place for nucleophilic attack.
- Then Proton is lost and consequently, Chlorine anion is removed from the molecule to give neutral product Benzanilide.
- We can see that HCl is also formed during the reaction. So presence of Sodium hydroxide not only catalyses the reaction but also neutralizes the effect of acid formed in the reaction.
- So, option (B) Benzanilide is correct.
Additional Information:
- This reaction involves formation of a C-N bond. We can categorize this reaction to Condensation Reactions.
-A Condensation reaction is a type of a reaction in which two molecules combine to give a product and small neutral molecules like water, Ammonia etc…
- Benzanilide is used as Fungicide and Pesticide as well.
- In this Schotten-Baumann reaction, Pyridine can also be used as a catalyst in place of Sodium hydroxide.
Note:
- Do not get confused between Benzoyl chloride, Benzyl Chloride, Benzal Chloride and Benzo Chloride whose structures are as shown below.

Here no suitable conditions or reagents are present for electrophilic aromatic substitution, So, do not consider it.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

Area of an Octagon Formula Explained Simply

Absolute Pressure Formula Explained: Key Equation & Examples

Central Angle of a Circle Formula Explained Quickly

Difference Between Vapor and Gas: JEE Main 2026

Difference Between Atom and Molecule: JEE Main 2026

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Jan 21 Shift 1 Question Papers with Solutions & Answer Keys – Detailed Day 1 Analysis

JEE Main Marks vs Percentile 2026: Calculate Percentile and Rank Using Marks

JEE Main 2026 Jan 22 Shift 1 Today Paper Live Analysis With Detailed Solutions

JEE Mains 2026 January 21 Shift 2 Question Paper with Solutions PDF - Complete Exam Analysis

JEE Main 2026 Jan 22 Shift 2 Today Paper Live Analysis With Detailed Solutions

Other Pages
Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages

One Day International Cricket

List of Highest T20 Scores in International Cricket

Valentine Week 2026: Complete List of Valentine Week Days & Meaning of Each Day

Makar Sankranti Wishes: Happy Makar Sankranti Wishes in Marathi, Hindi, Kannada, and English

What is the Full Form of UGC? Detailed Guide for Students




