
Of the four structural displays, which does outfit the octet rule description of the electron dot model developed by G.N. Lewis?
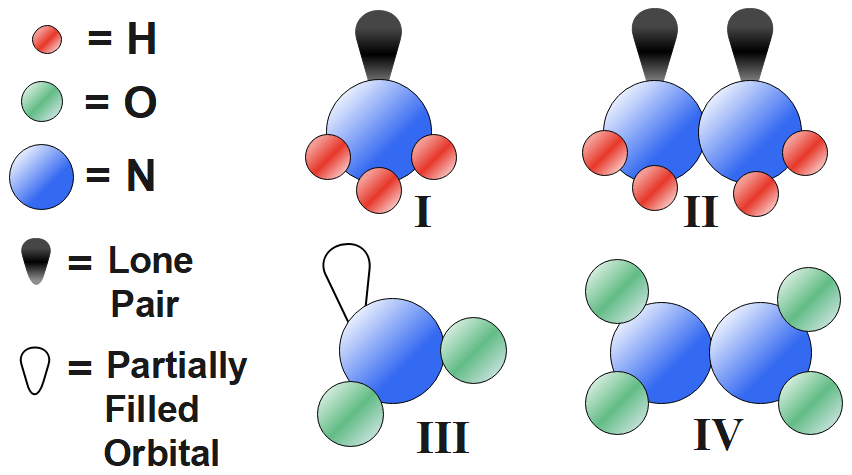
A.II
B.III
C.IV
D.All four fit the Lewis model.

Answer
508.5k+ views
Hint: We know that meanwhile drawing the Lewis dot structure, take only the valence shell electrons into account as they take part in the bonding. Also, consider the octet rule to fulfil the stability of the molecule formed.
Complete answer:
As the octet rule is fully satisfied, the number of bond pairs formed lead to decrease in the bond distance and thus, the bond strength increases making the diatom formed to be stable. Lewis dot structure also called electron dot structure shows its valence electrons of elements and describes the chemical bonding. They also display the total number of lone presents in each of the atoms. Lewis dot structures can be drawn if the molecular formula of the compound is known. Every element has a specific number of electrons present in its atomic orbitals.
Out of these electrons, only those participating in bond formation with another atom which is present in the outermost shell of the atom. These electrons which are present in the outermost orbital of an atom are called valence electrons. Any atom forms a bond with another atom to attain stability by completing its octet. And it is not necessary that each electron present in the valence shell takes part in octet formation. In some cases, it has been observed that one or more pairs of electrons in the valence shell remain unshared during bond formation. Such pairs of electrons in the outermost shell of an atom that remains unshared are known as lone pairs of electrons. In option III the central atom octet is not completed; it has only five electrons, one of partially filled orbital and four electrons which is in sharing with oxygen. So, it is not fit for the octet-rule description of the electron-dot model developed by G.N. Lewis.
Therefore, the correct answer is option B.
Note:
Remember that the nature of bond and position of atoms of the molecule which are connected in the molecule. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor forming a covalent bond because nitrogen shares their valence electrons to complete octet. Also, that only the valence electrons are considered while drawing Lewis does not structure and the electrons that do not belong to the outermost shell.
Complete answer:
As the octet rule is fully satisfied, the number of bond pairs formed lead to decrease in the bond distance and thus, the bond strength increases making the diatom formed to be stable. Lewis dot structure also called electron dot structure shows its valence electrons of elements and describes the chemical bonding. They also display the total number of lone presents in each of the atoms. Lewis dot structures can be drawn if the molecular formula of the compound is known. Every element has a specific number of electrons present in its atomic orbitals.
Out of these electrons, only those participating in bond formation with another atom which is present in the outermost shell of the atom. These electrons which are present in the outermost orbital of an atom are called valence electrons. Any atom forms a bond with another atom to attain stability by completing its octet. And it is not necessary that each electron present in the valence shell takes part in octet formation. In some cases, it has been observed that one or more pairs of electrons in the valence shell remain unshared during bond formation. Such pairs of electrons in the outermost shell of an atom that remains unshared are known as lone pairs of electrons. In option III the central atom octet is not completed; it has only five electrons, one of partially filled orbital and four electrons which is in sharing with oxygen. So, it is not fit for the octet-rule description of the electron-dot model developed by G.N. Lewis.
Therefore, the correct answer is option B.
Note:
Remember that the nature of bond and position of atoms of the molecule which are connected in the molecule. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor forming a covalent bond because nitrogen shares their valence electrons to complete octet. Also, that only the valence electrons are considered while drawing Lewis does not structure and the electrons that do not belong to the outermost shell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




