
On industrial scale ${H_2}{O_2}$ is prepared by autoxidation of:
A. 2-ethylanthraquinol
B. 2-ethylanthraquinone
C. 1-ethylanthraquinol
D. 1-ethyl anthraquinone
Answer
604.8k+ views
Hint: Try to recall that on an industrial scale ${H_2}{O_2}$ is prepared from an organic compound which is a derivative of anthraquinone and also, it is a pale yellow solid. Now, by using this you can easily find the correct option from the given options.
Complete step by step solution:
It is known to you that commercially ${H_2}{O_2}$ is prepared by autoxidation of an organic compound which is a derivative of anthraquinone.
The organic compound which is a derivative of anthraquinone is 2-ethylanthraquinol.
Hydrogen peroxide is prepared by autoxidation of 2-ethylanthraquinol. It involves oxidation of 2-ethylanthraquinol. The reaction is:
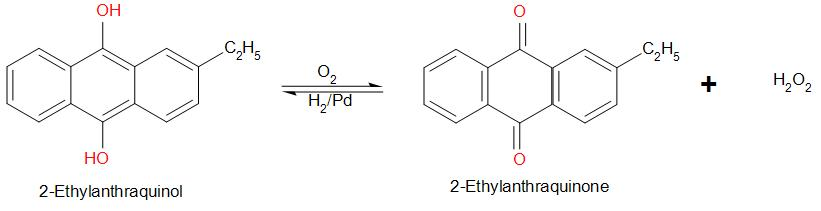
2-ethylanthraquinol is prepared by the reduction of 2-ethylanthraquinone by ${H_2}$ in the presence of palladium.
Therefore, from above we can easily conclude that option A is the correct option to the given question.
Additional information:
Preparation of ${H_2}{O_2}$ by autoxidation of 2-ethylanthraquinol:
First, air is passed through a mixture of 2-ethylanthraquinol mixture in cyclohexane and benzene.
It yields 2-ethylanthraquinone and hydrogen peroxide.
The product, 2-ethylanthraquinone is then reduced. For this, hydrogen under a pressure of 1-3 atmospheres at 4 degree Celsius is passed over 2-ethylanthraquinone in the presence of a Pd catalyst.
This gives back the reactant - 2-ethylanthraquinol.
The ${H_2}{O_2}$ is extracted with water and the solution of required concentration is obtained.
Note: It should be remembered to you that in the autoxidation of 2-ethylanthraquinol, only atmospheric oxygen and hydrogen are used which are inexpensive. Hence, this method is widely used for the preparation of hydrogen peroxide as it is quite cheap.
Complete step by step solution:
It is known to you that commercially ${H_2}{O_2}$ is prepared by autoxidation of an organic compound which is a derivative of anthraquinone.
The organic compound which is a derivative of anthraquinone is 2-ethylanthraquinol.
Hydrogen peroxide is prepared by autoxidation of 2-ethylanthraquinol. It involves oxidation of 2-ethylanthraquinol. The reaction is:

2-ethylanthraquinol is prepared by the reduction of 2-ethylanthraquinone by ${H_2}$ in the presence of palladium.
Therefore, from above we can easily conclude that option A is the correct option to the given question.
Additional information:
Preparation of ${H_2}{O_2}$ by autoxidation of 2-ethylanthraquinol:
First, air is passed through a mixture of 2-ethylanthraquinol mixture in cyclohexane and benzene.
It yields 2-ethylanthraquinone and hydrogen peroxide.
The product, 2-ethylanthraquinone is then reduced. For this, hydrogen under a pressure of 1-3 atmospheres at 4 degree Celsius is passed over 2-ethylanthraquinone in the presence of a Pd catalyst.
This gives back the reactant - 2-ethylanthraquinol.
The ${H_2}{O_2}$ is extracted with water and the solution of required concentration is obtained.
Note: It should be remembered to you that in the autoxidation of 2-ethylanthraquinol, only atmospheric oxygen and hydrogen are used which are inexpensive. Hence, this method is widely used for the preparation of hydrogen peroxide as it is quite cheap.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




