
On mixing a certain alkane with chlorine and irradiating it with U.V light it forms only one mono chloroalkane. This alkane could be
(a) Pentane
(b) Isopentane
(c) Neopentane
(d) Propane
Answer
546.3k+ views
Hint: In halogenation reaction, depending on the types of hydrogen atom present, the chemical compound on photochemical reaction gives single or multiple monochlorinated products.
Complete step by step answer:
Halogenation is the reaction where one or more than one hydrogen atom present in the chemical compound is replaced by a halogen group. It is a type of substitution reaction where the C-H bond is broken and a new C-X bond is formed.
In pentane five carbon atoms are present in a straight chain. It contains three types of the hydrogen atom which on monochlorination give three products. They are 1-chloropentane, 2-chloropentane and 3-chloropentane.
In isopentane five carbon atoms are present out of which one is in branched form. It contains four types of a hydrogen atom which is present which on monochlorination gives four products. They are: 1-chloro-3-methylbutane, 2-chloro-3-methylbutane, 2-chloro-2-methylbutane and 1-chloro-2-methylbutane.
In neopentane five carbon atoms are present which is doubly branched. It contains only one type of hydrogen atom which on monochlorination gives one product which is 1-chloro-2,2-dimethylbutane.
In propane three carbon atoms are present in a straight chain. It contains two types of hydrogen atom which on monochlorination give two products. They are 1-chloropropane and 2-chloropropane.
The reaction of neopentane with chlorine is shown below.
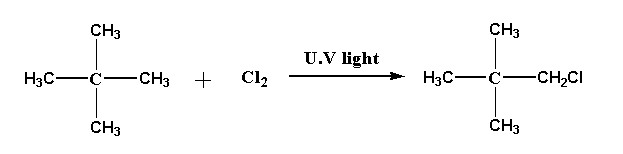
Neopentane reacts with chlorine in presence of U.V light to form 2,2-dimethyl 1-chloropropane.
So, the correct answer is Option C.
Note: Isopentane, neopentane, and n-pentane are the isomer of pentane as they have the same molecular formula but they differ from each other by their structural representation.
Complete step by step answer:
Halogenation is the reaction where one or more than one hydrogen atom present in the chemical compound is replaced by a halogen group. It is a type of substitution reaction where the C-H bond is broken and a new C-X bond is formed.
In pentane five carbon atoms are present in a straight chain. It contains three types of the hydrogen atom which on monochlorination give three products. They are 1-chloropentane, 2-chloropentane and 3-chloropentane.
In isopentane five carbon atoms are present out of which one is in branched form. It contains four types of a hydrogen atom which is present which on monochlorination gives four products. They are: 1-chloro-3-methylbutane, 2-chloro-3-methylbutane, 2-chloro-2-methylbutane and 1-chloro-2-methylbutane.
In neopentane five carbon atoms are present which is doubly branched. It contains only one type of hydrogen atom which on monochlorination gives one product which is 1-chloro-2,2-dimethylbutane.
In propane three carbon atoms are present in a straight chain. It contains two types of hydrogen atom which on monochlorination give two products. They are 1-chloropropane and 2-chloropropane.
The reaction of neopentane with chlorine is shown below.

Neopentane reacts with chlorine in presence of U.V light to form 2,2-dimethyl 1-chloropropane.
So, the correct answer is Option C.
Note: Isopentane, neopentane, and n-pentane are the isomer of pentane as they have the same molecular formula but they differ from each other by their structural representation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




