
On ozonolysis of cyclohexene followed by reaction with zinc dust and water gives a compound $E$. Compound $E$ on further treatment with aqueous $KOH$ yield compound $F$. Compound $F$ is:
Answer
569.4k+ views
Hint:
In cyclohexene there is one double bond. On ozonolysis of cyclohexene followed by reaction with zinc dust and water break this double bond and two aldehyde groups are formed by carbon atom sharing this double bond. When this compound is further treated with aqueous $KOH$, then aldol condensation takes place between the alpha-hydrogen of one aldehyde group with another aldehyde group.
Complete step by step answer:
Cyclohexene has one double bond. And we know that on ozonolysis followed by reaction with zinc dust and water break the double bond from a compound with aldehyde groups on carbon atoms that share that double bond.
Cyclohexene is converted into a linear compound with aldehyde at both the ends which is compound $E$, after the above process done with cyclohexane.
Now we have two aldehyde groups in compound E and when compound $E$ is treated further with KOH then a reaction takes place which is famously known as aldol condensation reaction. In aldol condensation, carbon of one aldehyde group forms a bond with alpha carbon of another aldehyde group. And this is shown in figure below:
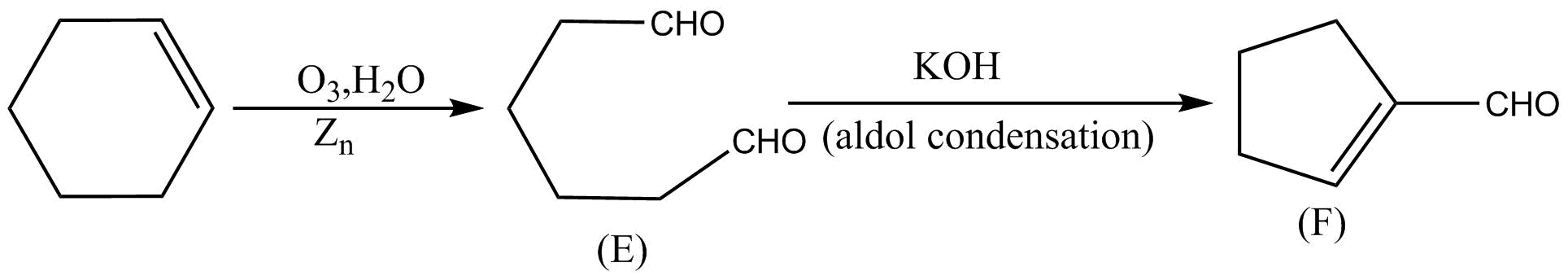
And in the above diagram we can see that after aldol condensation we get another compound $F$ which is the same as the compound in image in option A.
Hence the correct answer is option A.
Note:Ozonolysis process is used for oxidation of a compound and it also breaks double bonds present in alkenes. Aldol condensation occurs only when there is alpha hydrogen on an aldehyde group. Here in the above reaction, we can see that ozonolysis divides the compound by breaking a bond and aldol condensation combines the compound by making bond.
In cyclohexene there is one double bond. On ozonolysis of cyclohexene followed by reaction with zinc dust and water break this double bond and two aldehyde groups are formed by carbon atom sharing this double bond. When this compound is further treated with aqueous $KOH$, then aldol condensation takes place between the alpha-hydrogen of one aldehyde group with another aldehyde group.
Complete step by step answer:
Cyclohexene has one double bond. And we know that on ozonolysis followed by reaction with zinc dust and water break the double bond from a compound with aldehyde groups on carbon atoms that share that double bond.
Cyclohexene is converted into a linear compound with aldehyde at both the ends which is compound $E$, after the above process done with cyclohexane.
Now we have two aldehyde groups in compound E and when compound $E$ is treated further with KOH then a reaction takes place which is famously known as aldol condensation reaction. In aldol condensation, carbon of one aldehyde group forms a bond with alpha carbon of another aldehyde group. And this is shown in figure below:

And in the above diagram we can see that after aldol condensation we get another compound $F$ which is the same as the compound in image in option A.
Hence the correct answer is option A.
Note:Ozonolysis process is used for oxidation of a compound and it also breaks double bonds present in alkenes. Aldol condensation occurs only when there is alpha hydrogen on an aldehyde group. Here in the above reaction, we can see that ozonolysis divides the compound by breaking a bond and aldol condensation combines the compound by making bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




