
On reaction of chlorobenzene with acetyl chloride in presence of anhydrous \[AlC{l_3}\] the major product formed is.
Answer
520.8k+ views
Hint: We know that A Friedel-Crafts response is a natural coupling response including an electrophilic fragrant replacement that is utilized for the connection of substituents to sweet-smelling rings. The two essential sorts of Friedel-Crafts responses are the alkylation and acylation responses. These responses were created in the year \[1877\] by the French scientific expert Charles Friedel and the American physicist James Crafts.
Complete answer:
The Friedel-Crafts acylation response includes the expansion of an acyl gathering to a fragrant ring. Ordinarily, this is finished by utilizing a corrosive chloride \[\left( {R - \left( {C = O} \right) - {\text{ }}Cl} \right)\] and a Lewis corrosive impetus like\[AlC{l_3}\] . In a Friedel-Crafts acylation response, the sweet-smelling ring is changed into a ketone.
An acid anhydride can be utilized as an option in contrast to the acyl halide in Friedel-Crafts acylations. The halogen having a place with the acyl halide shapes a complex with the Lewis corrosive, producing a profoundly electrophilic acylium particle, which has an overall recipe of \[RC{O^ + }\] and is settled by reverberation.
The given reaction is Friedel Crafts acylation reaction and here para item structure as significant item due to ortho, para coordinating nature of \[C{l^ - }\] group. The major product formed by the given reaction is 2-chloroacetophenone.
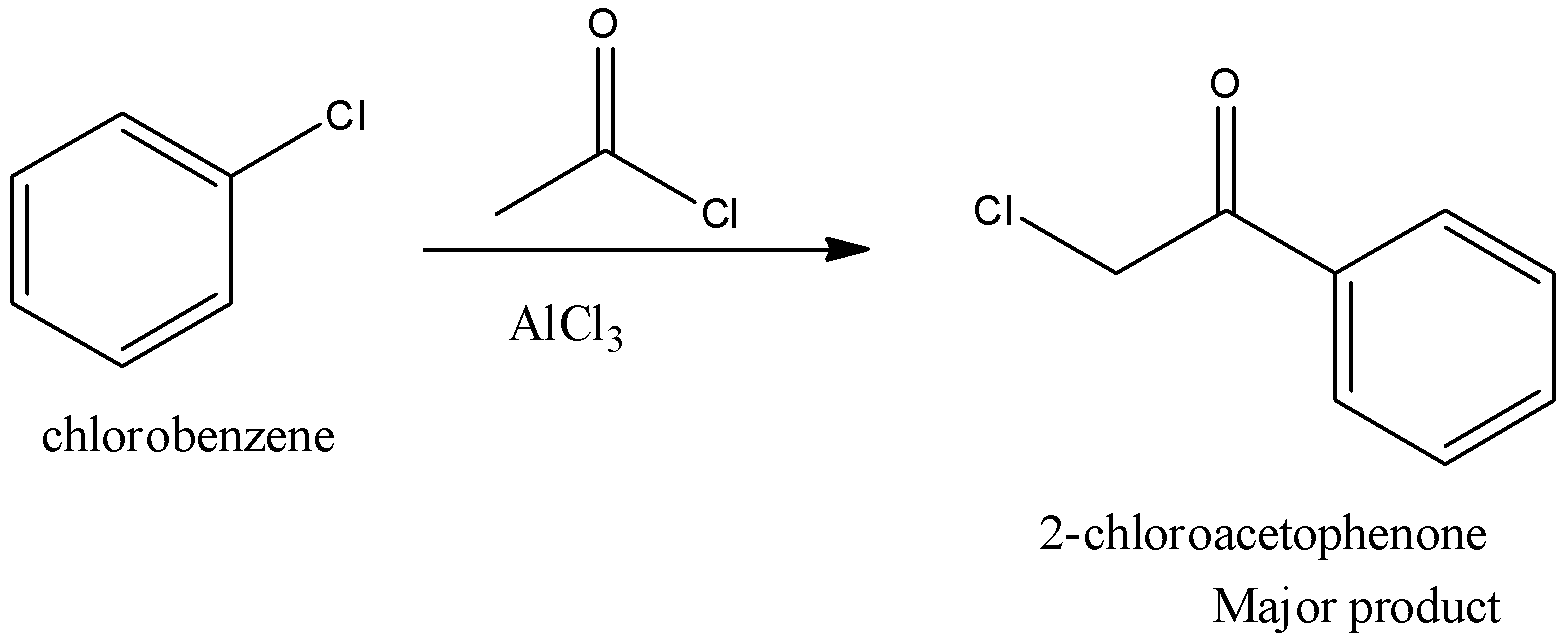
This reaction is drawn on chem.
Note:
We need to remember that the Friedel-Crafts Alkylation alludes to the supplanting of a fragrant proton with an alkyl bunch. This is done through an electrophilic assault on the sweet-smelling ring with the assistance of a carbocation. The Friedel-Crafts alkylation response is a technique for producing alkyl benzenes by utilizing alkyl halides as reactants. \[FeC{l_3}\] Or \[AlC{l_3}\] is utilized in this response to shape a carbocation by working with the evacuation of the halide. The subsequent carbocation goes through an improvement prior to continuing with the alkylation response.
Complete answer:
The Friedel-Crafts acylation response includes the expansion of an acyl gathering to a fragrant ring. Ordinarily, this is finished by utilizing a corrosive chloride \[\left( {R - \left( {C = O} \right) - {\text{ }}Cl} \right)\] and a Lewis corrosive impetus like\[AlC{l_3}\] . In a Friedel-Crafts acylation response, the sweet-smelling ring is changed into a ketone.
An acid anhydride can be utilized as an option in contrast to the acyl halide in Friedel-Crafts acylations. The halogen having a place with the acyl halide shapes a complex with the Lewis corrosive, producing a profoundly electrophilic acylium particle, which has an overall recipe of \[RC{O^ + }\] and is settled by reverberation.
The given reaction is Friedel Crafts acylation reaction and here para item structure as significant item due to ortho, para coordinating nature of \[C{l^ - }\] group. The major product formed by the given reaction is 2-chloroacetophenone.

This reaction is drawn on chem.
Note:
We need to remember that the Friedel-Crafts Alkylation alludes to the supplanting of a fragrant proton with an alkyl bunch. This is done through an electrophilic assault on the sweet-smelling ring with the assistance of a carbocation. The Friedel-Crafts alkylation response is a technique for producing alkyl benzenes by utilizing alkyl halides as reactants. \[FeC{l_3}\] Or \[AlC{l_3}\] is utilized in this response to shape a carbocation by working with the evacuation of the halide. The subsequent carbocation goes through an improvement prior to continuing with the alkylation response.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




