
On reductive ozonolysis what is the product?
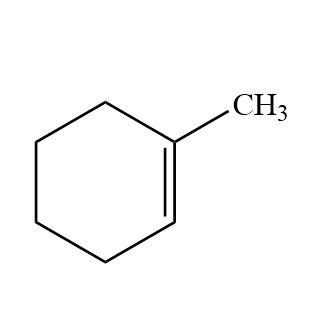

Answer
582.3k+ views
Hint
Ozonolysis is described as a kind of cycloaddition that breaks bonds. In this reaction, cleavage of pi bond will take place in such a way that the final products give two carbonyl groups.
Complete step by step solution
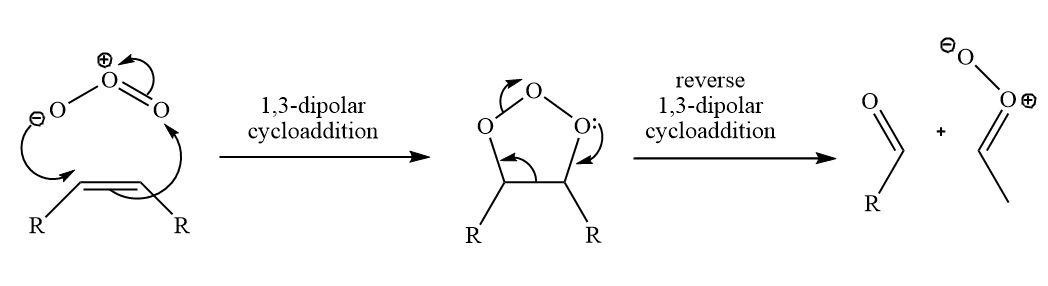
In the first reaction 1,3-dipolar cycloaddition reaction will take place.
The reagent is ozone,${{\rm{O}}_{\rm{3}}}$.Ozone is a symmetrical bent molecule with a central positively charged oxygen atom and two terminal oxygen atoms that share a negative charge. It is a 1,3-dipole and does typical 1,3-dipolar cycloadditions with alkenes. The product is a very unstable compound. The ${\rm{O - O}}$ single bond (bond energy\[{\rm{140 kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\]) is a very weak bond much weaker than the N–O bond (\[{\rm{180 kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\]). We have been described as weak in previous examples—and this heterocycle has two of them. It immediately decomposes by a reverse 1,3-dipolar cycloaddition.
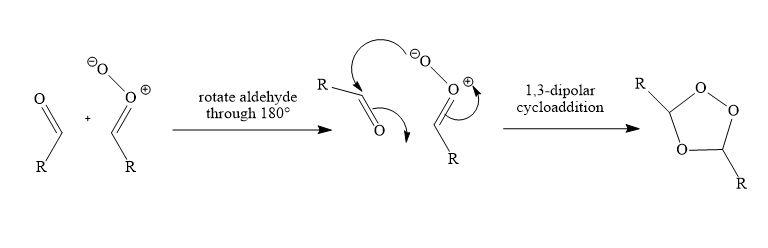
The products are a simple aldehyde on the left and a new, rather unstable looking molecule—1,3-dipole known as a carbonyl oxide on the right. At least it no longer has any true ${\rm{O - O}}$ single bonds (the one that looks like a single bond is part of a delocalized system like the one in ozone). Being a 1,3-dipole, it now adds to the aldehyde in a third cycloaddition step. It might just add back the way it came, but it much prefers to add in the other way round with the nucleophilic oxyanion attacking the carbon atom of the carbonyl group like this.
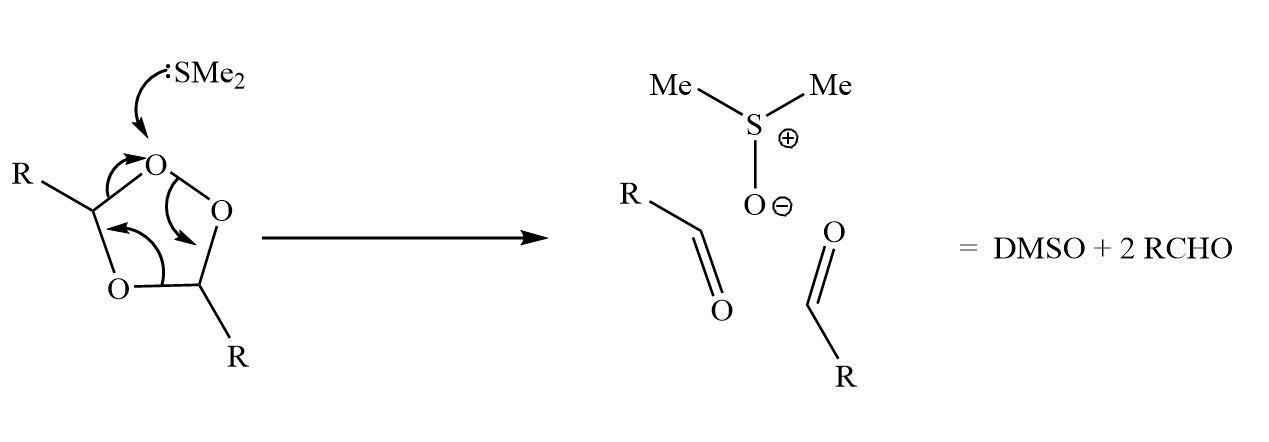
The product is ozonide which is not stable. The ozonide is explosive in nature. So, it readily decomposes by dimethyl sulfide to yield molecules of aldehyde.
Hence, our final product is shown below.
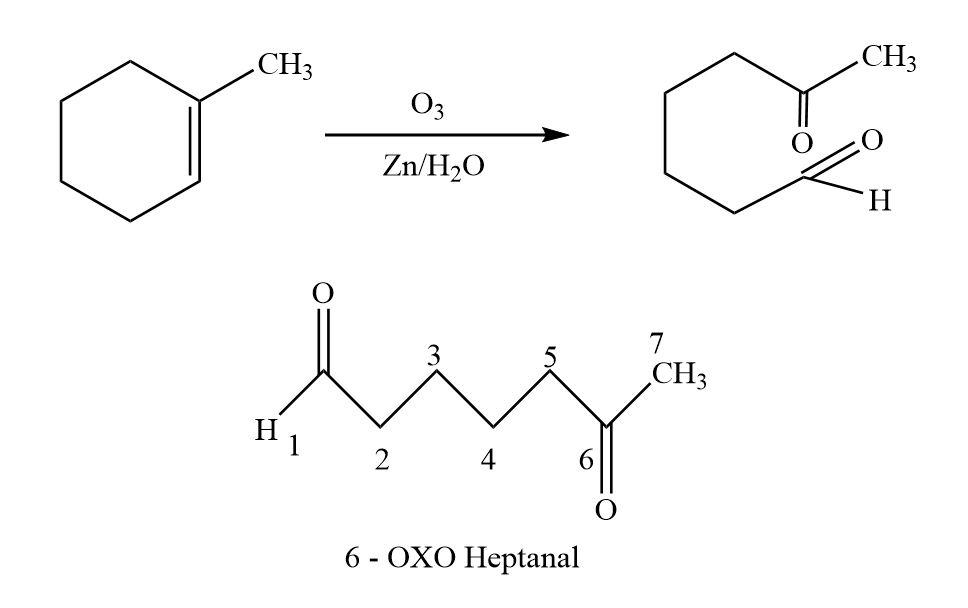
Note
The oxidative ozonolysis requires hydrogen peroxide in place of Zinc and it results in a carboxylic form.
Ozonolysis is described as a kind of cycloaddition that breaks bonds. In this reaction, cleavage of pi bond will take place in such a way that the final products give two carbonyl groups.
Complete step by step solution
In the first reaction 1,3-dipolar cycloaddition reaction will take place.
The reagent is ozone,${{\rm{O}}_{\rm{3}}}$.Ozone is a symmetrical bent molecule with a central positively charged oxygen atom and two terminal oxygen atoms that share a negative charge. It is a 1,3-dipole and does typical 1,3-dipolar cycloadditions with alkenes. The product is a very unstable compound. The ${\rm{O - O}}$ single bond (bond energy\[{\rm{140 kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\]) is a very weak bond much weaker than the N–O bond (\[{\rm{180 kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\]). We have been described as weak in previous examples—and this heterocycle has two of them. It immediately decomposes by a reverse 1,3-dipolar cycloaddition.

The products are a simple aldehyde on the left and a new, rather unstable looking molecule—1,3-dipole known as a carbonyl oxide on the right. At least it no longer has any true ${\rm{O - O}}$ single bonds (the one that looks like a single bond is part of a delocalized system like the one in ozone). Being a 1,3-dipole, it now adds to the aldehyde in a third cycloaddition step. It might just add back the way it came, but it much prefers to add in the other way round with the nucleophilic oxyanion attacking the carbon atom of the carbonyl group like this.

The product is ozonide which is not stable. The ozonide is explosive in nature. So, it readily decomposes by dimethyl sulfide to yield molecules of aldehyde.

Hence, our final product is shown below.

Note
The oxidative ozonolysis requires hydrogen peroxide in place of Zinc and it results in a carboxylic form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




