
One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having molecular mass of ${\text{44g/mol}}{\text{.}}$ The alkene is:
A.Propane
B.1-butene
C.2-butene
D.Ethene
Answer
594k+ views
Hint:
There are many important methods for preparation of aldehydes and ketones. One such important method is the reductive ozonolysis of alkenes. In this method, alkenes react with ozone to form ozonides which upon reductive cleavage with zinc dust and water will give aldehydes and ketones. Symmetrically disubstituted alkenes give aldehydes other than formaldehyde and tetrasubstituted alkenes give ketones. Unsymmetrical alkenes can give both aldehydes and ketones.
Complete step by step answer:
The first given option is propane which is an alkane. It is not an alkene. So, it does not give any aldehyde on ozonolysis. So, option A is incorrect.
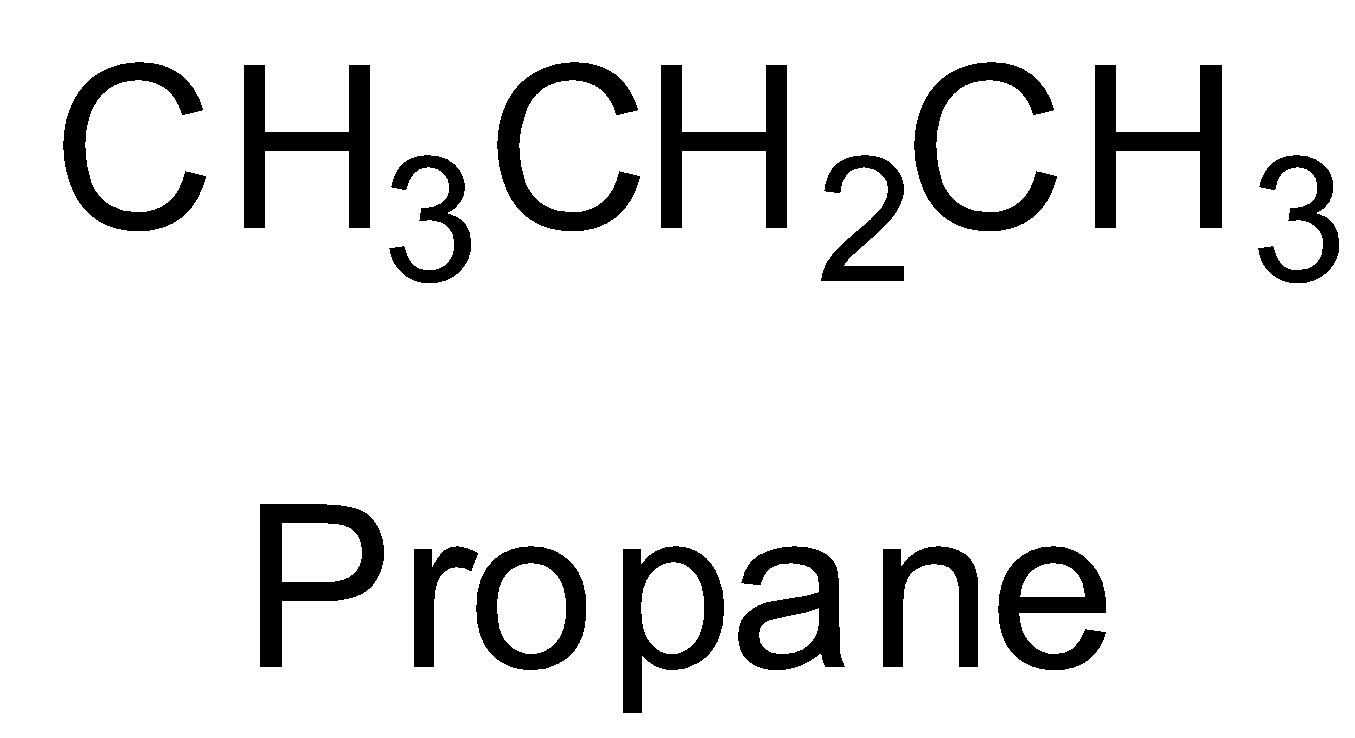
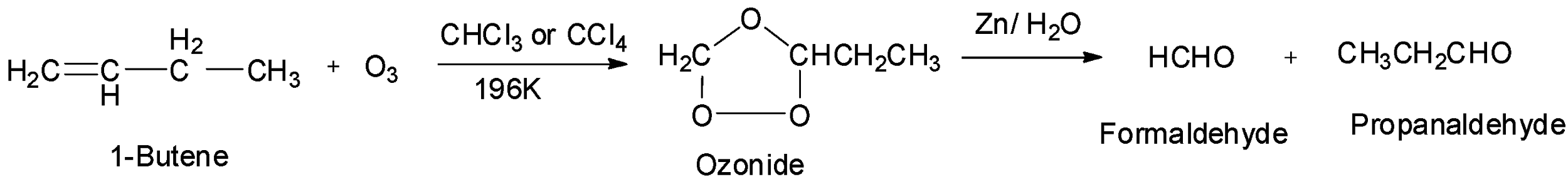
The second given option is 1-butene which is an unsymmetrical alkene. Therefore, it will undergo ozonolysis and give two different aldehydes, formaldehyde and propanaldehyde. Hence, option B is incorrect.
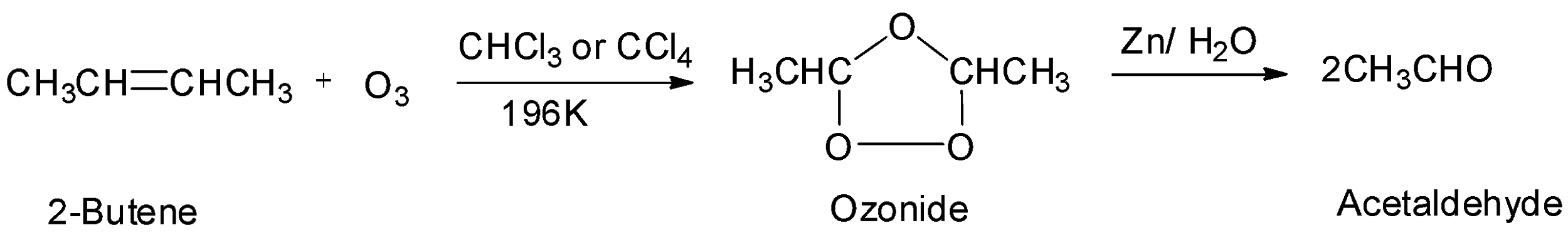
The third option is 2-butene which is a symmetrical alkene. Therefore, it will undergo ozonolysis and will give two moles of an aldehyde. The reaction will be: From the reaction, it is seen that 2-butene gives two moles of acetaldehyde whose molecular mass equals to ${\text{44g/mol}}{\text{.}}$ This matches the given data. Hence, the symmetrical alkene is 2-butene and the aldehyde formed is acetaldehyde. So, the correct option is option C.
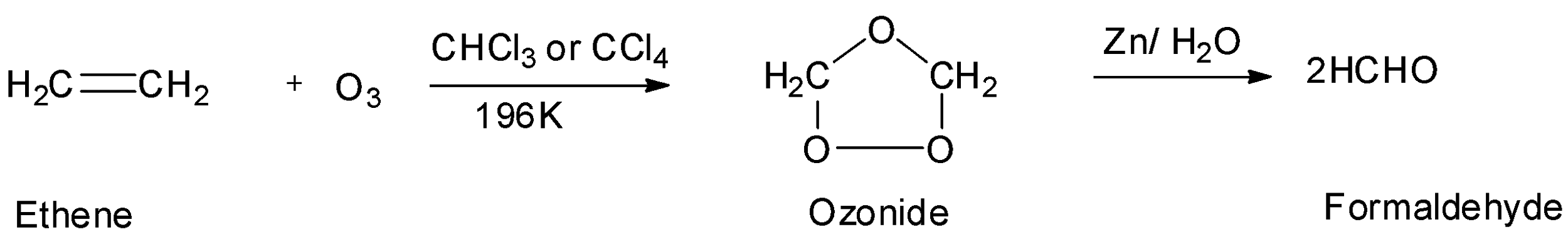
The fourth option is ethene or ethylene which is also symmetrical. So, it will also undergo ozonolysis to give an aldehyde which is formaldehyde. But the molecular mass of formaldehyde is not ${\text{44g/mol}}{\text{.}}$ So, the symmetrical alkene is not ethene. Hence, option D is incorrect.
Hence option C is correct.
Note:
Alkynes also undergo ozonolysis to give acid anhydrides or diketones. A reducing agent is not needed in this case and the exact mechanism is also not known. If the reaction is carried out in the presence of water, the anhydride will hydrolyze to give two carboxylic acids.
There are many important methods for preparation of aldehydes and ketones. One such important method is the reductive ozonolysis of alkenes. In this method, alkenes react with ozone to form ozonides which upon reductive cleavage with zinc dust and water will give aldehydes and ketones. Symmetrically disubstituted alkenes give aldehydes other than formaldehyde and tetrasubstituted alkenes give ketones. Unsymmetrical alkenes can give both aldehydes and ketones.
Complete step by step answer:
The first given option is propane which is an alkane. It is not an alkene. So, it does not give any aldehyde on ozonolysis. So, option A is incorrect.
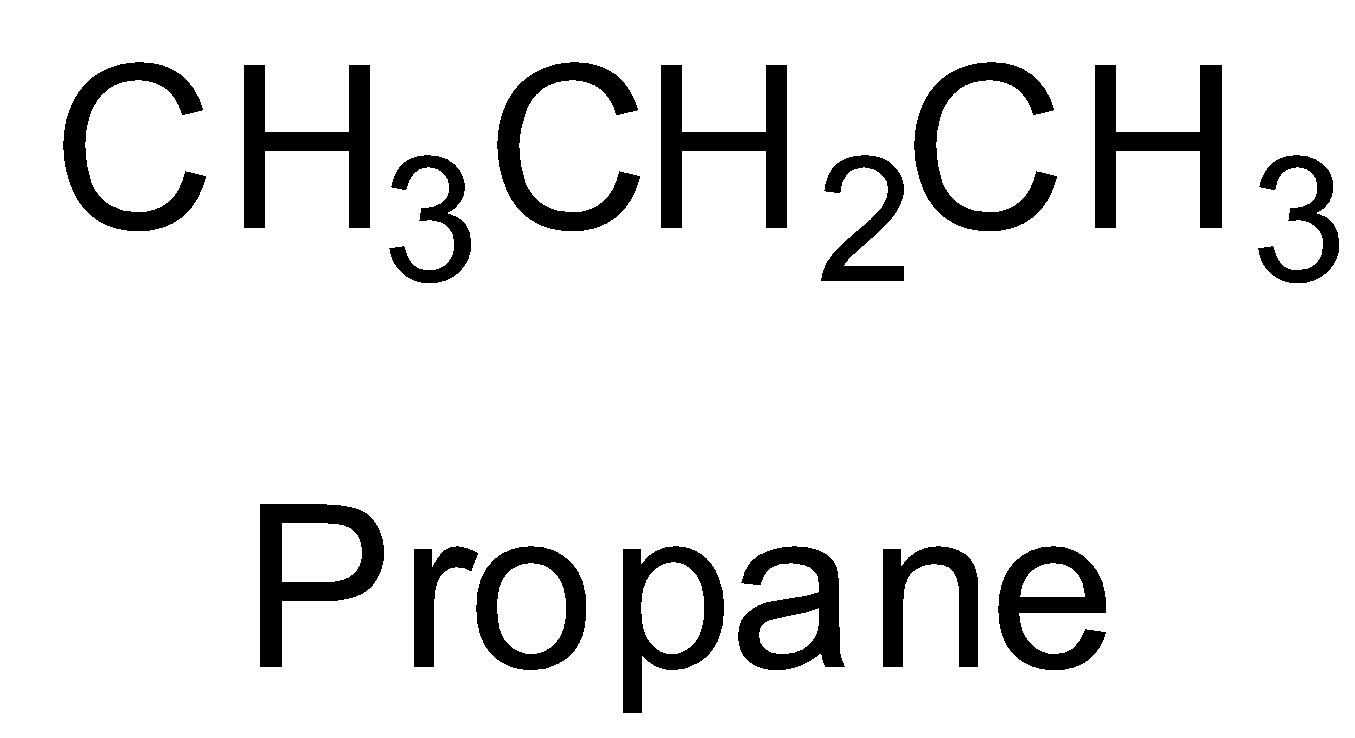
The second given option is 1-butene which is an unsymmetrical alkene. Therefore, it will undergo ozonolysis and give two different aldehydes, formaldehyde and propanaldehyde. Hence, option B is incorrect.

The third option is 2-butene which is a symmetrical alkene. Therefore, it will undergo ozonolysis and will give two moles of an aldehyde. The reaction will be: From the reaction, it is seen that 2-butene gives two moles of acetaldehyde whose molecular mass equals to ${\text{44g/mol}}{\text{.}}$ This matches the given data. Hence, the symmetrical alkene is 2-butene and the aldehyde formed is acetaldehyde. So, the correct option is option C.

The fourth option is ethene or ethylene which is also symmetrical. So, it will also undergo ozonolysis to give an aldehyde which is formaldehyde. But the molecular mass of formaldehyde is not ${\text{44g/mol}}{\text{.}}$ So, the symmetrical alkene is not ethene. Hence, option D is incorrect.

Hence option C is correct.
Note:
Alkynes also undergo ozonolysis to give acid anhydrides or diketones. A reducing agent is not needed in this case and the exact mechanism is also not known. If the reaction is carried out in the presence of water, the anhydride will hydrolyze to give two carboxylic acids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




