
Why is o-nitrophenol steam volatile whereas p-nitrophenol has a higher boiling point. Explain.
Answer
580.8k+ views
Hint: Basically, in case to o-nitrophenol, there is intramolecular hydrogen bonding whereas in case of p-nitrophenol there is intermolecular hydrogen bonding. Moreover, intramolecular hydrogen bonding occurs when two functional groups of a molecule can form hydrogen bonds with each other.
Complete step by step answer:
Nitro phenols are the compounds of the formula $HO{C_6}{H_{5 - X}}{(N{O_2})_X}$ . These are more acidic than phenol. Further, there are three isomeric nitro phenols which are listed below.
a)o-nitrophenol
b)m-nitrophenol
c)p-nitrophenol
Now, o-nitrophenol also known as 2- nitrophenol is a light-yellow solid with a peculiar sweet smell whereas p-nitrophenol or 4 nitrophenol is a colorless to light yellow solid with very little odor.2-Nitrophenol is a member in which one of the hydrogens that is ortho to hydroxyl group has been replaced by a nitro group. Their structures are as shown:
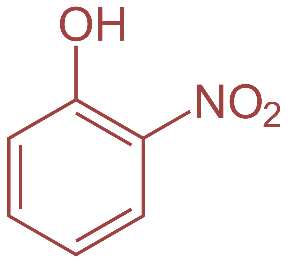
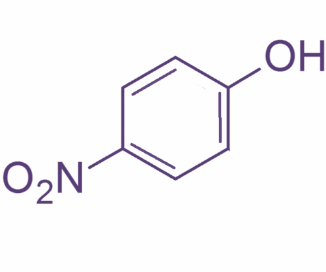
So, basically o-nitrophenol is steam volatile whereas p-nitrophenol has a higher boiling point. This is because o-nitrophenol has intermolecular hydrogen bonding leading to less association between adjacent molecules and hence lower boiling point whereas in case of p-nitrobenzene there is intermolecular hydrogen bonding which leads to more association. This further requires more energy to break the hydrogen bonds and thus leading to higher boiling point.
Note: Nitro phenols are poisonous in nature. They generally contaminate the soil near fabric factories and military plants. They should be handled with care to avoid any serious circumstance. Moreover, inhalation or ingestion may cause headache, nausea, blue color in lips and ears, drowsiness, cyanosis and eye irritation.
Complete step by step answer:
Nitro phenols are the compounds of the formula $HO{C_6}{H_{5 - X}}{(N{O_2})_X}$ . These are more acidic than phenol. Further, there are three isomeric nitro phenols which are listed below.
a)o-nitrophenol
b)m-nitrophenol
c)p-nitrophenol
Now, o-nitrophenol also known as 2- nitrophenol is a light-yellow solid with a peculiar sweet smell whereas p-nitrophenol or 4 nitrophenol is a colorless to light yellow solid with very little odor.2-Nitrophenol is a member in which one of the hydrogens that is ortho to hydroxyl group has been replaced by a nitro group. Their structures are as shown:


So, basically o-nitrophenol is steam volatile whereas p-nitrophenol has a higher boiling point. This is because o-nitrophenol has intermolecular hydrogen bonding leading to less association between adjacent molecules and hence lower boiling point whereas in case of p-nitrobenzene there is intermolecular hydrogen bonding which leads to more association. This further requires more energy to break the hydrogen bonds and thus leading to higher boiling point.
Note: Nitro phenols are poisonous in nature. They generally contaminate the soil near fabric factories and military plants. They should be handled with care to avoid any serious circumstance. Moreover, inhalation or ingestion may cause headache, nausea, blue color in lips and ears, drowsiness, cyanosis and eye irritation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




