
What is osmosis? How does it help in our daily life?
Answer
516.9k+ views
Hint: The unconstrained net development of dissolvable particles through a specifically penetrable layer into a district of higher solute fixation, toward the path that will in general adjust the solute focuses on the two sides.
Complete answer
It is a vital process in biological systems, as biological membranes are semipermeable. Generally, these membranes are impermeable to huge and polar molecules, for example, ions, polysaccharides, and proteins, while being permeable to nonpolar or hydrophobic atoms such as lipids additionally as to small molecules like oxygen, carbon dioxide, nitrogen, and gas. It is the process where the solvents move from a region of higher to lower concentration gradient through a semipermeable membrane. It draws water from the soil through the roots of the plants. Plants concentrate solutes in their root cells by transport, and water enters the roots by osmosis. Osmosis is additionally liable for controlling the movement of guard cells.
It can easily be demonstrated through an experiment of potato where the potato slices are dipped in a hypertonic solution (high salt concentration). The water present inside the potato comes out resulting in its shrinkage, this process is termed as 'turgor pressure'.
When the membrane features a volume of pure water on each side, water molecules pass in and call in each direction at precisely the same rate. Thus the net water movement across the layer is zero.
The mechanism liable for driving osmosis has commonly been represented in biology and chemistry texts as either the dilution of water by a solute (resulting in a lower concentration of water on the higher solute focus side of the film and in this way dispersion of water along a fixation angle) or by a solute's appreciation for water. They are later refuted.
The diffusion model of osmosis is rendered untenable by the very fact that osmosis can drive water across a membrane toward a better concentration of water. The "bound water" model is refuted by the very fact that osmosis is independent of the dimensions of the solute molecules.
Note:
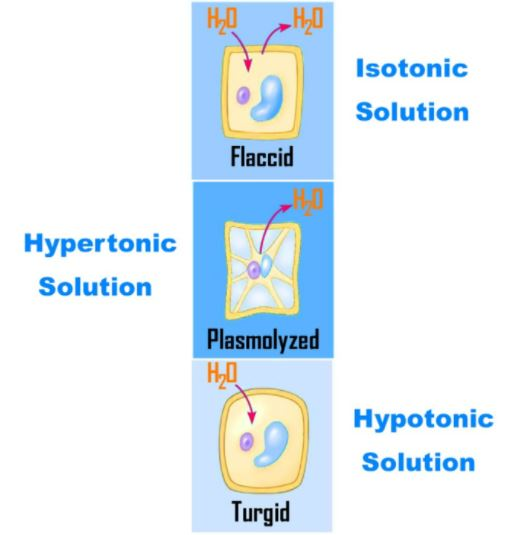
Osmosis is mainly of three types, Hypotonic- it contains less number of particles or solutes compared to the cell. This results in the cells swell as the waster moves inside them and may even cause bursting.
Hypertonic-it contains a greater number of solute particles than normally present in a cell. This results in the shrinkage of the cells as the water moves outside.
Isotonic – it contains the same number of solute molecules present in the cell. So, there will be no net movement of water in or out from the cell.
Complete answer
It is a vital process in biological systems, as biological membranes are semipermeable. Generally, these membranes are impermeable to huge and polar molecules, for example, ions, polysaccharides, and proteins, while being permeable to nonpolar or hydrophobic atoms such as lipids additionally as to small molecules like oxygen, carbon dioxide, nitrogen, and gas. It is the process where the solvents move from a region of higher to lower concentration gradient through a semipermeable membrane. It draws water from the soil through the roots of the plants. Plants concentrate solutes in their root cells by transport, and water enters the roots by osmosis. Osmosis is additionally liable for controlling the movement of guard cells.
It can easily be demonstrated through an experiment of potato where the potato slices are dipped in a hypertonic solution (high salt concentration). The water present inside the potato comes out resulting in its shrinkage, this process is termed as 'turgor pressure'.
When the membrane features a volume of pure water on each side, water molecules pass in and call in each direction at precisely the same rate. Thus the net water movement across the layer is zero.
The mechanism liable for driving osmosis has commonly been represented in biology and chemistry texts as either the dilution of water by a solute (resulting in a lower concentration of water on the higher solute focus side of the film and in this way dispersion of water along a fixation angle) or by a solute's appreciation for water. They are later refuted.
The diffusion model of osmosis is rendered untenable by the very fact that osmosis can drive water across a membrane toward a better concentration of water. The "bound water" model is refuted by the very fact that osmosis is independent of the dimensions of the solute molecules.
Note:
Osmosis is mainly of three types, Hypotonic- it contains less number of particles or solutes compared to the cell. This results in the cells swell as the waster moves inside them and may even cause bursting.
Hypertonic-it contains a greater number of solute particles than normally present in a cell. This results in the shrinkage of the cells as the water moves outside.
Isotonic – it contains the same number of solute molecules present in the cell. So, there will be no net movement of water in or out from the cell.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




