
Out of ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{4}}}{{\text{O}}_{\text{6}}}{\text{,}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{5}}}$ and ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$ peroxy acids are:
A.\[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{4}}}{{\text{O}}_{\text{6}}}\]
B.${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{4}}}{{\text{O}}_{\text{6}}}{\text{,}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{5}}}$
C.${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{5}}}{\text{,}}{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$
D.${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$
Answer
583.8k+ views
Hint: Peroxy acids are sometimes all known as peracid. They are a group of compounds which have –OOH group instead of an –OH group or the compound that have a peroxy bond, i.e. an Oxygen-oxygen single covalent bond (-O-O-). Some of the examples of peroxy acids are Peroxyacetic acid, Perchloric acid.
Complete step by step answer:
To which one of the given Sulphur compounds falls under the category of peroxy acids let us look into the structure of each one of these:
${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}$: This acid is known as thiosulfuric acid.
Structure:
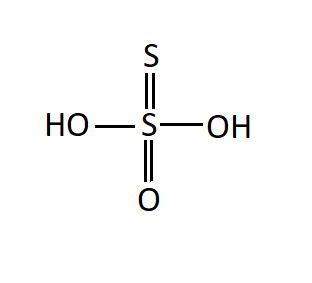
Since there is no oxygen-oxygen single covalent bond, it is not a peroxy acid.
${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{6}}}$: It is known as Tetrathionic acid.
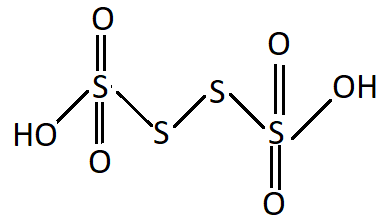
Structure:
As you can see there is no oxygen-oxygen single covalent bond it cannot be regarded as a peroxy acid.
${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{5}}}$: This acid is known as Peroxymonosulphuric acid.
Structure:
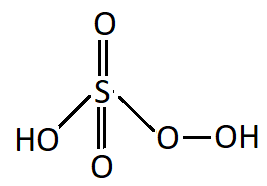
As it can be seen the molecule of Peroxymonosulfuric acid contains an oxygen-oxygen single covalent bond, it is a peroxy acid of sulphur.
${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$: This acid is known as Peroxydisulphuric acid.
Structure:
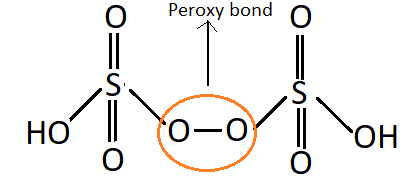
As it can be seen in the structure this molecule of acid contains an oxygen-oxygen single covalent bond, it falls under the category of peroxy acids of sulphur.
Hence, after looking at all the structures of given acids of sulphur, we can conclude that out of the four given acids, ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{5}}}{\text{,}}{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$ are the peroxy acids.
And thus option C is the correct answer.
Note:There are some very important uses of Peroxy acids. As these acids contain an extra oxygen atom, they can be used for:
Oxidising a compound: they act as oxidising agents in many organic reactions, where they themselves get reduced.
These compounds find a wide variety of uses in organic chemistry: to convert alkenes into epoxides, ketones into esters and amines into nitro compounds are some of the applications.
Complete step by step answer:
To which one of the given Sulphur compounds falls under the category of peroxy acids let us look into the structure of each one of these:
${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}$: This acid is known as thiosulfuric acid.
Structure:

Since there is no oxygen-oxygen single covalent bond, it is not a peroxy acid.
${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{6}}}$: It is known as Tetrathionic acid.

Structure:
As you can see there is no oxygen-oxygen single covalent bond it cannot be regarded as a peroxy acid.
${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{5}}}$: This acid is known as Peroxymonosulphuric acid.
Structure:

As it can be seen the molecule of Peroxymonosulfuric acid contains an oxygen-oxygen single covalent bond, it is a peroxy acid of sulphur.
${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$: This acid is known as Peroxydisulphuric acid.
Structure:

As it can be seen in the structure this molecule of acid contains an oxygen-oxygen single covalent bond, it falls under the category of peroxy acids of sulphur.
Hence, after looking at all the structures of given acids of sulphur, we can conclude that out of the four given acids, ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{5}}}{\text{,}}{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$ are the peroxy acids.
And thus option C is the correct answer.
Note:There are some very important uses of Peroxy acids. As these acids contain an extra oxygen atom, they can be used for:
Oxidising a compound: they act as oxidising agents in many organic reactions, where they themselves get reduced.
These compounds find a wide variety of uses in organic chemistry: to convert alkenes into epoxides, ketones into esters and amines into nitro compounds are some of the applications.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




