
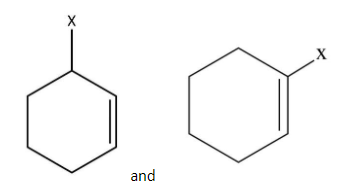
Out of which is an example of vinylic halide?

Answer
573.9k+ views
Hint: As we know that, vinylic carbon is \[S{p^2}\] hybridized. In this type of molecule, the pi-bond is attached by a halogen atom present in the molecule. Actually vinyl groups are identified by the position of the double bond with the carbon atom to which a group is attached.
Complete Step by step answer: he halogen atom of benzyl halide is bonded with a hybrid carbon. There is a small amount of s-character in this carbon, but it is less electronegative. The halogen carbon with s-character quantity is \[33\;\% \], making it more electronegative than the former. Nucleophilic substitution reaction requires a high benzylic halide and is more reactive towards nucleophilic substitution reaction. Vinyl halide is more stable since the C-X bond is stronger due to more double bond character.
Allylic halide is a compound in which a halogen atom is connected to Allylic carbon. Allylic halide undergoes faster \[S{N^2}\] reaction than that of tertiary alkyl halide. The reason is simple \[S{N^2}\] reaction is a concerted step where a backside attack of nucleophilic occurs that means nucleophilic attack to the reacting C-center away from the leaving group.
This is vinylic halide.
Vinyl halide is inert towards both \[S{N^1}\] and \[S{N^2}\] mechanisms. The \[S{N^2}\] mechanism is not favoured for three reasons.
The substituent around a double bond are within the same plane, therefore an \[S{N^2}\] would give steric hindrance.
It means that the nucleophilic would have to attack a high electron density region with the double bond, including electronic repulsion.
The transition state of this \[S{N^2}\] reaction would be sp hybrid carbon which is not energetically favorable.
Note: Organic chemistry is versatile, so with some adoption a \[S{N^1}\] on a vinyl carbon is feasible. Vinyl is basically a group formed from alkene. There are two types of vinyl halide, one is substituted vinyl halide and second one is unsubstituted vinyl halide.
Vinyl halides are more stable due to the presence of double bond character C and hydrogen.
Complete Step by step answer: he halogen atom of benzyl halide is bonded with a hybrid carbon. There is a small amount of s-character in this carbon, but it is less electronegative. The halogen carbon with s-character quantity is \[33\;\% \], making it more electronegative than the former. Nucleophilic substitution reaction requires a high benzylic halide and is more reactive towards nucleophilic substitution reaction. Vinyl halide is more stable since the C-X bond is stronger due to more double bond character.
Allylic halide is a compound in which a halogen atom is connected to Allylic carbon. Allylic halide undergoes faster \[S{N^2}\] reaction than that of tertiary alkyl halide. The reason is simple \[S{N^2}\] reaction is a concerted step where a backside attack of nucleophilic occurs that means nucleophilic attack to the reacting C-center away from the leaving group.

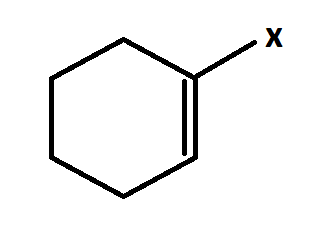
This is vinylic halide.
Vinyl halide is inert towards both \[S{N^1}\] and \[S{N^2}\] mechanisms. The \[S{N^2}\] mechanism is not favoured for three reasons.
The substituent around a double bond are within the same plane, therefore an \[S{N^2}\] would give steric hindrance.
It means that the nucleophilic would have to attack a high electron density region with the double bond, including electronic repulsion.
The transition state of this \[S{N^2}\] reaction would be sp hybrid carbon which is not energetically favorable.
Note: Organic chemistry is versatile, so with some adoption a \[S{N^1}\] on a vinyl carbon is feasible. Vinyl is basically a group formed from alkene. There are two types of vinyl halide, one is substituted vinyl halide and second one is unsubstituted vinyl halide.
Vinyl halides are more stable due to the presence of double bond character C and hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




