
Oxidation of ethanedioate ions by acidified manganate (VII) ions is very slow at room temperature.
\[2Mn{{O}_{4}}^{-}+5{{C}_{2}}{{O}_{4}}^{2-}+16{{H}^{+}}\to 2M{{n}^{3+}}+10C{{O}_{2}}+8{{H}_{2}}O\]\[2Mn{{O}_{4}}^{-}+5{{C}_{2}}{{O}_{4}}^{2-}+16{{H}^{+}}\to 2M{{n}^{2+}}+10C{{O}_{2}}+8{{H}_{2}}O\]
\[M{{n}^{2+}}\]ions catalyse this reaction.
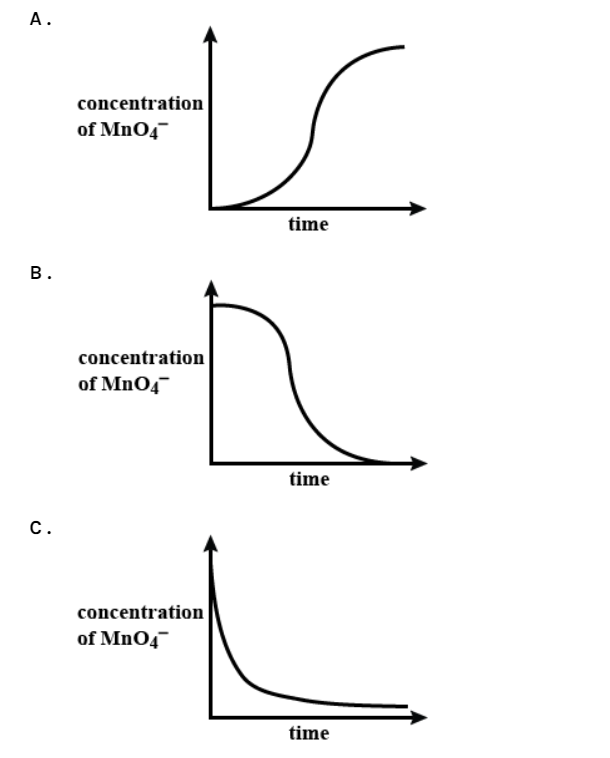
Which graph shows how the concentration of acidified manganate (VII) ions varies after ethanedioate ions are added?

Answer
596.1k+ views
Hint: As the catalyst of this reaction (\[M{{n}^{2+}}\]) is produced in the course of this reaction, this would help steadily increase the rate of reaction. Pick an answer with this logic in mind.
Complete step by step solution:
Let us analyse this reaction in a gradual manner to ensure that we understand this phenomenon in its entirety.
The reaction between manganate (VII) ions and ethanedioate ions at room temperature is fairly slow initially but quickens as the reaction proceeds. Manganese (II) ions, \[M{{n}^{2+}}\]are formed as the reaction proceeds and they act as an auto-catalyst. Once a small amount of \[M{{n}^{2+}}\] ions have formed, they can react with \[Mn{{O}_{4}}^{-}\] ions to form \[M{{n}^{3+}}\] ions as an intermediate species. These then react with the \[{{C}_{2}}{{O}_{4}}^{2-}\] ions to reform \[M{{n}^{2+}}\].
As a few \[M{{n}^{2+}}\]ions are formed, the rate increases and proceeds following first-order kinetics with respect to the manganate (VII) ions. Adding \[M{{n}^{2+}}\]ions at the beginning of the reaction results in a smooth decrease in concentration with time from the outset. As an extension task the students can go on to use their plots of concentration of \[Mn{{O}_{4}}^{-}\]ions against time to determine the rate of the reaction for different concentrations of \[Mn{{O}_{4}}^{-}\]ions.
A plot of log(rate) against log[\[Mn{{O}_{4}}^{-}\]]should then be a straight line with a gradient of 1 indicating that the reaction is first order with respect to manganate(VII) ions.
Therefore, the answer to this question would be (B).
Note: If the concentration of any one of the reactants is plotted against time, then it has to decrease as time goes on. While if the same happens with the product compounds, then with time their concentration would increase. The only exception to this case would be the reactions in dynamic equilibrium.
The phenomenon involved here is known as autocatalysis, which means one of the products of this reaction behaves as a catalyst. Bear in mind, that the speed of reaction will gradually increase if this is the case. If the reaction is already provided with that particular product right from the start, then the answer to this question would have been nearly similar to option (C).
Complete step by step solution:
Let us analyse this reaction in a gradual manner to ensure that we understand this phenomenon in its entirety.
The reaction between manganate (VII) ions and ethanedioate ions at room temperature is fairly slow initially but quickens as the reaction proceeds. Manganese (II) ions, \[M{{n}^{2+}}\]are formed as the reaction proceeds and they act as an auto-catalyst. Once a small amount of \[M{{n}^{2+}}\] ions have formed, they can react with \[Mn{{O}_{4}}^{-}\] ions to form \[M{{n}^{3+}}\] ions as an intermediate species. These then react with the \[{{C}_{2}}{{O}_{4}}^{2-}\] ions to reform \[M{{n}^{2+}}\].
As a few \[M{{n}^{2+}}\]ions are formed, the rate increases and proceeds following first-order kinetics with respect to the manganate (VII) ions. Adding \[M{{n}^{2+}}\]ions at the beginning of the reaction results in a smooth decrease in concentration with time from the outset. As an extension task the students can go on to use their plots of concentration of \[Mn{{O}_{4}}^{-}\]ions against time to determine the rate of the reaction for different concentrations of \[Mn{{O}_{4}}^{-}\]ions.
A plot of log(rate) against log[\[Mn{{O}_{4}}^{-}\]]should then be a straight line with a gradient of 1 indicating that the reaction is first order with respect to manganate(VII) ions.
Therefore, the answer to this question would be (B).
Note: If the concentration of any one of the reactants is plotted against time, then it has to decrease as time goes on. While if the same happens with the product compounds, then with time their concentration would increase. The only exception to this case would be the reactions in dynamic equilibrium.
The phenomenon involved here is known as autocatalysis, which means one of the products of this reaction behaves as a catalyst. Bear in mind, that the speed of reaction will gradually increase if this is the case. If the reaction is already provided with that particular product right from the start, then the answer to this question would have been nearly similar to option (C).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




