
Why is the oxygen dissociation curve S shaped?
Answer
492k+ views
Hint: The difference between oxygen saturation, which is the percentage of haemoglobin bound to oxygen, and partial pressure of oxygen in the blood, which is the amount of oxygen dissolved in the blood, is vital to understand. The oxyhemoglobin dissociation curve is a useful tool for describing the link between these two key concepts.
Complete answer:
The oxyhemoglobin dissociation curve is a crucial tool for understanding how oxygen is transported and released by blood. Hemoglobin, a protein molecule found inside red blood cells, is primarily responsible for transporting oxygen throughout the body. Oxygen can also be delivered throughout the body by dissolving in blood plasma, but this dissolved fraction accounts for only a small portion of the total oxygen transported in the bloodstream. Only 2% of oxygen in the bloodstream dissolves directly in the plasma component of blood, compared to 98 percent of oxygen linked to haemoglobin in the protein-bound condition.
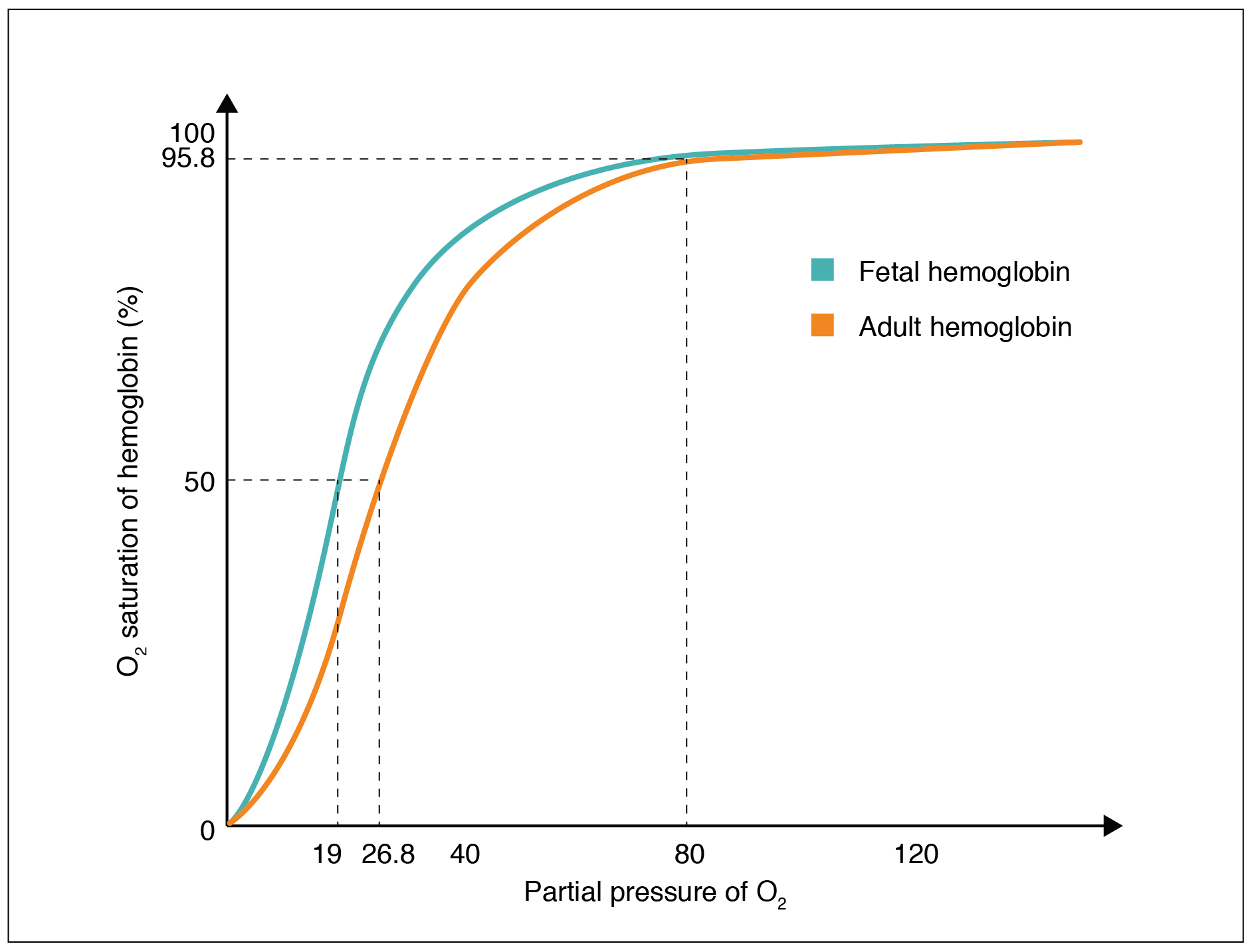
As the oxygen partial pressure rises, more molecules bind until the maximum amount that can be bound is reached. As the haemoglobin reaches this limit, very little additional binding occurs, and the curve flattens out as the haemoglobin becomes oxygen-saturated. As a result, the curve is sigmoidal or S-shaped.
The local prevalent carbon dioxide partial pressure (partial pressure of carbon dioxide), pH, and temperature are all known to alter the oxygen dissociation curve (ODC) in the past. Higher partial pressure of carbon dioxide, greater acidity (lower pH), and higher temperature push the curve to the right..
Temperature increases shift the curve to the right, whereas temperature decreases shift the curve to the left. The connection between oxygen and haemoglobin is denatured as the temperature rises, increasing the amount of oxygen and haemoglobin while decreasing the concentration of oxyhaemoglobin.
Note:
Hemoglobin (Hb) is a molecule made up of protein globin and a nonprotein component called haem that is responsible for transporting oxygen in the blood. Hemoglobin is made up of four subunits: two alpha and two beta subunits, each with its own heme group and globin chain. At its heart, the heme group has an iron atom in a ferrous state that attaches to one oxygen molecule, allowing one haemoglobin tetramer to bind four oxygen molecules.
Complete answer:
The oxyhemoglobin dissociation curve is a crucial tool for understanding how oxygen is transported and released by blood. Hemoglobin, a protein molecule found inside red blood cells, is primarily responsible for transporting oxygen throughout the body. Oxygen can also be delivered throughout the body by dissolving in blood plasma, but this dissolved fraction accounts for only a small portion of the total oxygen transported in the bloodstream. Only 2% of oxygen in the bloodstream dissolves directly in the plasma component of blood, compared to 98 percent of oxygen linked to haemoglobin in the protein-bound condition.
As the oxygen partial pressure rises, more molecules bind until the maximum amount that can be bound is reached. As the haemoglobin reaches this limit, very little additional binding occurs, and the curve flattens out as the haemoglobin becomes oxygen-saturated. As a result, the curve is sigmoidal or S-shaped.
The local prevalent carbon dioxide partial pressure (partial pressure of carbon dioxide), pH, and temperature are all known to alter the oxygen dissociation curve (ODC) in the past. Higher partial pressure of carbon dioxide, greater acidity (lower pH), and higher temperature push the curve to the right..
Temperature increases shift the curve to the right, whereas temperature decreases shift the curve to the left. The connection between oxygen and haemoglobin is denatured as the temperature rises, increasing the amount of oxygen and haemoglobin while decreasing the concentration of oxyhaemoglobin.

Note:
Hemoglobin (Hb) is a molecule made up of protein globin and a nonprotein component called haem that is responsible for transporting oxygen in the blood. Hemoglobin is made up of four subunits: two alpha and two beta subunits, each with its own heme group and globin chain. At its heart, the heme group has an iron atom in a ferrous state that attaches to one oxygen molecule, allowing one haemoglobin tetramer to bind four oxygen molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




