
Oxygen molecule is
A.diamagnetic with no-unpaired electrons
B.diamagnetic with two unpaired electrons
C.paramagnetic with two unpaired electrons
D.paramagnetic with no unpaired electrons
Answer
584.4k+ views
Hint: To answer this question, recall the concept of molecular orbital theory. After a combination of atomic orbitals fill each molecular orbital in order of their energies. Now check whether there is the existence of unpaired or paired electrons.
Formula used: \[\dfrac{{{{\text{N}}_{\text{b}}} - {{\text{N}}_{\text{a}}}}}{2}\] for bond order where ${N_b}$ is no. of electrons in bonding atomic orbital and ${N_a}$is the no. of electrons in an anti-bonding atomic orbital
Complete step by step answer:
From the atomic number of oxygen that each oxygen atom has 8 electrons. Therefore, there are 16 electrons in the oxygen molecule. Now as per molecular orbital theory the combination of atomic orbitals of the two atoms, ten molecular orbitals are formed.
The filling of these orbitals takes place in the order of increasing energy and is governed by the following rules:
The molecular orbitals are arranged in the increasing order of their energies. The molecular orbital with the lowest energy is filled first as per the Aufbau principle.
Each molecular orbital can accommodate a maximum of two electrons with opposite spins as per Pauli's exclusion principle.
If there are two or more molecular orbitals of the same energy, these are the first singly filled and then pairing starts as per the Hund's rule.
Draw the MOT diagram of \[{O_2}\]:
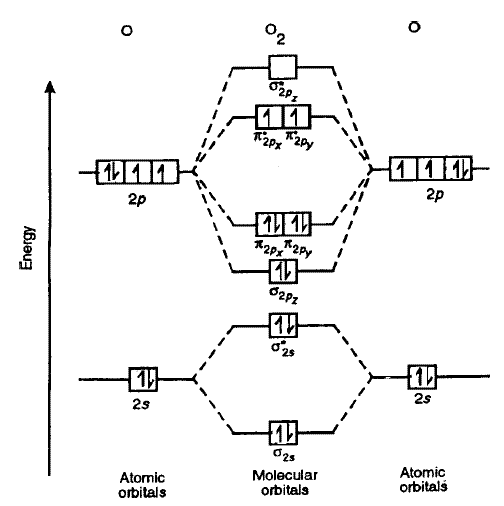
As can be seen from the MOT of \[{O_2}\], The highest occupied molecular orbital has 2 unpaired electrons therefore it is paramagnetic. Also, the bond order can be calculated as:
\[\dfrac{{{{\text{N}}_{\text{b}}} - {{\text{N}}_{\text{a}}}}}{2}\ = \dfrac{{10 - 6}}{2} = 2\]
We can see that \[{O_2}\]is paramagnetic with 2 unpaired electrons.
Therefore, we can conclude that the correct answer to this question is option C.
Note:
While drawing MOT diagrams the important points to be kept in mind are:
1.Law of conservation of orbitals: The number of molecular orbitals generated is equal to the number of creating atomic orbitals.
2.As the overlap between two atomic orbitals increases, the difference in energy between the resulting bonding and antibonding molecular orbitals increases with the increase in overlap of the atomic orbitals.
3.The interaction between atomic orbitals is greatest when they have the same energy.
Formula used: \[\dfrac{{{{\text{N}}_{\text{b}}} - {{\text{N}}_{\text{a}}}}}{2}\] for bond order where ${N_b}$ is no. of electrons in bonding atomic orbital and ${N_a}$is the no. of electrons in an anti-bonding atomic orbital
Complete step by step answer:
From the atomic number of oxygen that each oxygen atom has 8 electrons. Therefore, there are 16 electrons in the oxygen molecule. Now as per molecular orbital theory the combination of atomic orbitals of the two atoms, ten molecular orbitals are formed.
The filling of these orbitals takes place in the order of increasing energy and is governed by the following rules:
The molecular orbitals are arranged in the increasing order of their energies. The molecular orbital with the lowest energy is filled first as per the Aufbau principle.
Each molecular orbital can accommodate a maximum of two electrons with opposite spins as per Pauli's exclusion principle.
If there are two or more molecular orbitals of the same energy, these are the first singly filled and then pairing starts as per the Hund's rule.
Draw the MOT diagram of \[{O_2}\]:

As can be seen from the MOT of \[{O_2}\], The highest occupied molecular orbital has 2 unpaired electrons therefore it is paramagnetic. Also, the bond order can be calculated as:
\[\dfrac{{{{\text{N}}_{\text{b}}} - {{\text{N}}_{\text{a}}}}}{2}\ = \dfrac{{10 - 6}}{2} = 2\]
We can see that \[{O_2}\]is paramagnetic with 2 unpaired electrons.
Therefore, we can conclude that the correct answer to this question is option C.
Note:
While drawing MOT diagrams the important points to be kept in mind are:
1.Law of conservation of orbitals: The number of molecular orbitals generated is equal to the number of creating atomic orbitals.
2.As the overlap between two atomic orbitals increases, the difference in energy between the resulting bonding and antibonding molecular orbitals increases with the increase in overlap of the atomic orbitals.
3.The interaction between atomic orbitals is greatest when they have the same energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




