
Ozonolysis of an organic compound gives formaldehyde as one of the products. This confirms the presence of
(A) two ethylenic double bonds
(B) a vinyl group
(C) as isopropyl group
(D) an acetylenic triple bond
Answer
578.1k+ views
Hint: Ozonolysis mechanism proceeds via an oxidative cleavage reaction. The ozone not only breaks the carbon pi bond but also the carbon-carbon sigma bond. It involves the attack of ozone on the given reactant to form an ozonide.
Complete step by step answer: Ozonolysis is an organic reaction where the unsaturated bonds of alkenes, alkynes, or azo compounds are cleaved with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl group while azo compounds form nitrosamines.
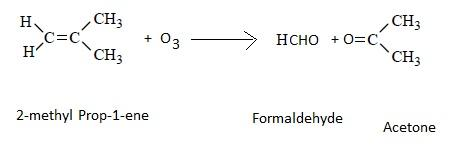
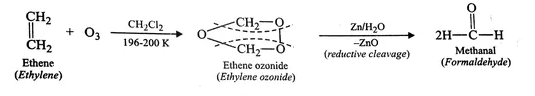
Presence of one vinyl (or ethenyl) group gives formaldehyde as one of the products in ozonolysis. CH2=CH- on ozonolysis will give HCHO.The formation of ozonolysis product involves formation of ozonide intermediate. Ozonide is highly unstable and explosive so it is reduced in situ by use of zinc metal.
Hence option B is the correct answer.
Note: The steps of ozonolysis are as follows:
1.The electrophilic addition of ozone to the carbon-carbon bond forms the molozonide intermediate which is quite unstable. Due to this unstable nature, the molozonide continues reacting – breaking apart to form a carbonyl molecule and a carbonyl oxide molecule.
2.The carbonyl molecule and the carbonyl oxide molecule formed in step 1 rearrange themselves, reforming to create a more stable ozonide intermediate. This ozonide intermediate can be subjected to either an oxidative workup or a reductive workup. The oxidative workup will give carboxylic acid as the product whereas the reductive workup will yield aldehydes or ketones.
Complete step by step answer: Ozonolysis is an organic reaction where the unsaturated bonds of alkenes, alkynes, or azo compounds are cleaved with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl group while azo compounds form nitrosamines.

Presence of one vinyl (or ethenyl) group gives formaldehyde as one of the products in ozonolysis. CH2=CH- on ozonolysis will give HCHO.The formation of ozonolysis product involves formation of ozonide intermediate. Ozonide is highly unstable and explosive so it is reduced in situ by use of zinc metal.

Hence option B is the correct answer.
Note: The steps of ozonolysis are as follows:
1.The electrophilic addition of ozone to the carbon-carbon bond forms the molozonide intermediate which is quite unstable. Due to this unstable nature, the molozonide continues reacting – breaking apart to form a carbonyl molecule and a carbonyl oxide molecule.
2.The carbonyl molecule and the carbonyl oxide molecule formed in step 1 rearrange themselves, reforming to create a more stable ozonide intermediate. This ozonide intermediate can be subjected to either an oxidative workup or a reductive workup. The oxidative workup will give carboxylic acid as the product whereas the reductive workup will yield aldehydes or ketones.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




