
Ozonolysis of o-xylene gives:
a.) Glyoxal
b.) Methylglyoxal
c.) Dimethyl Glyoxal
d.) All of the above
Answer
585.6k+ views
Hint: To answer this first you should draw the structure o-xylene. Ozonolysis is the process of cleaving the double bond by the formation of a five membered ring. Here the ring will be formed including the carbon atoms included in the double bonding. This will lead to the formation of more than one product.
Complete step by step answer:
Before answering the question, let us understand what an ozonolysis reaction is.
To break a double bond we need to carry the process by two successive oxidations. First oxidation is of the –pi bond and the second is of the –sigma bond. This is called double oxidation.
We can carry out this oxidation in a single step by ozone.
We know that ozone is a symmetrical bent molecule with a central positively charged oxygen atom and the other two oxygen atoms i.e. the terminal oxygen atoms share a negative charge. Ozone is unstable and we generate it immediately before use from oxygen using a device called ozonizer. Then it is bubbled into the reaction mixture.
We add $Os{{O}_{4}}$ to alkenes and it is added by a cyclic mechanism making a five membered ring with the three oxygen atoms. This structure is extremely unstable and collapses by breaking a weak O – O bond and a C – C sigma bond but gains two strong –C=O bonds in this process.
We get an aldehyde and an unstable carbonyl oxide as products. When we add a mild reducing agent to this, the carbonyl oxide is also converted to aldehyde thus giving us two aldehydes.
This process of bond cleaving in alkenes is known as ozonolysis. Now let us discuss the ozonolysis of o-xylene. We know that o-xylene is the other name for o-dimethylbenzene. Therefore, we can draw its structure as-
Now, let us see the mechanism of ozonolysis of the above compound.
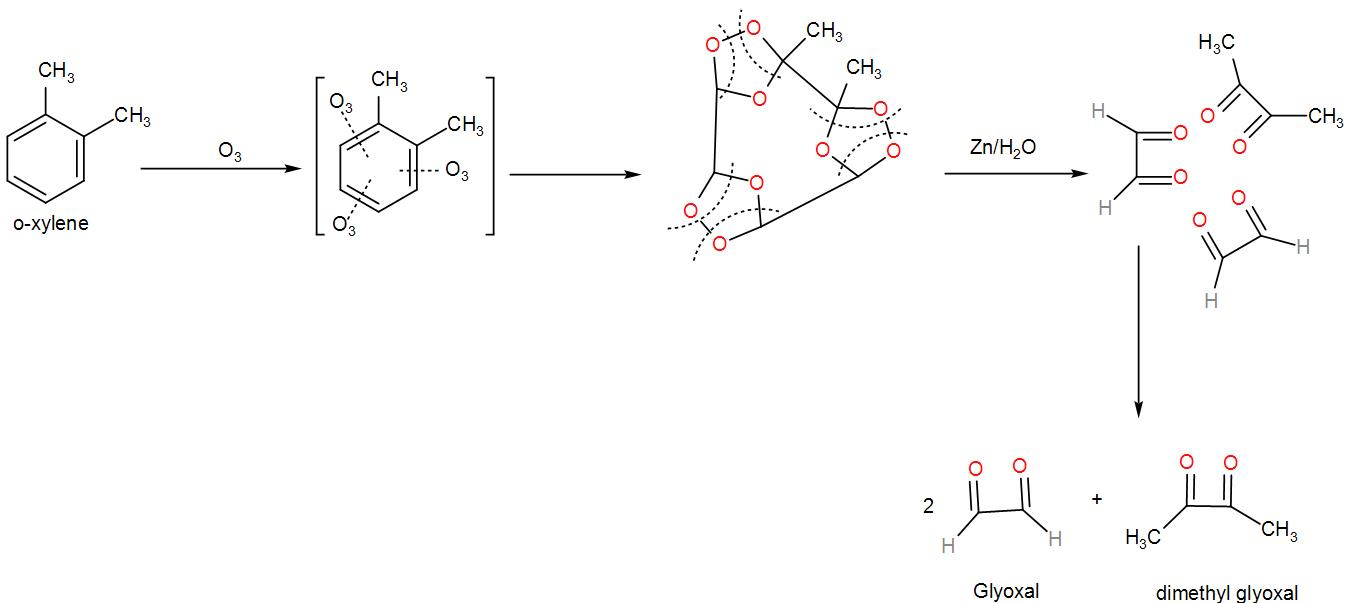
Now, if the bond cleaving was different and included the two methyl groups in two different five-membered rings instead of the same ring as above then, we would receive a mixture of products which contained methylglyoxal and glyoxal. We can draw it as-
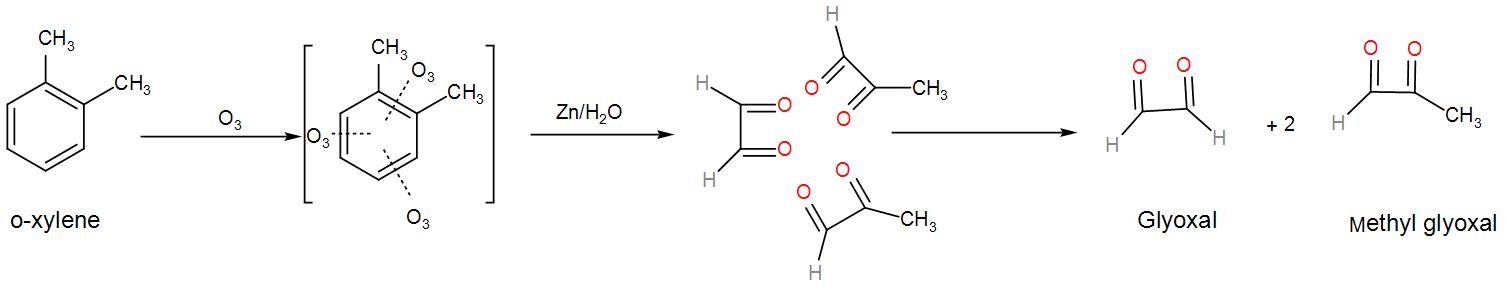
We can understand from the above discussion that o-xylene will give a mixture of glyoxal, methylglyoxal, and dimethyl glyoxal since there are two resonating structures of xylene.
These products suggest that benzene has 3 double bonds in the ring and their positions are as shown. (These resonant structures are obtained from two Kekule structures).
This is also a test to prove that benzene (in this case o-xylene) is a hybrid of two Kekule structures.
So, the correct answer is “Option D”.
Note: Here we saw that 1,2-dimethylbenzene (o-xylene) on ozonolysis gave glyoxal, methylglyoxal, and dimethyl glyoxal in 3:2:1 molar ratio. This proves the existence of two resonance structures for benzene and its derivatives.
Kekule structure: A Lewis structure in which bonded electron pairs in covalent bonds are shown as lines. The most famous Kekule structures are what we would now call the two most significant resonance contributors of benzene.
Complete step by step answer:
Before answering the question, let us understand what an ozonolysis reaction is.
To break a double bond we need to carry the process by two successive oxidations. First oxidation is of the –pi bond and the second is of the –sigma bond. This is called double oxidation.
We can carry out this oxidation in a single step by ozone.
We know that ozone is a symmetrical bent molecule with a central positively charged oxygen atom and the other two oxygen atoms i.e. the terminal oxygen atoms share a negative charge. Ozone is unstable and we generate it immediately before use from oxygen using a device called ozonizer. Then it is bubbled into the reaction mixture.
We add $Os{{O}_{4}}$ to alkenes and it is added by a cyclic mechanism making a five membered ring with the three oxygen atoms. This structure is extremely unstable and collapses by breaking a weak O – O bond and a C – C sigma bond but gains two strong –C=O bonds in this process.
We get an aldehyde and an unstable carbonyl oxide as products. When we add a mild reducing agent to this, the carbonyl oxide is also converted to aldehyde thus giving us two aldehydes.
This process of bond cleaving in alkenes is known as ozonolysis. Now let us discuss the ozonolysis of o-xylene. We know that o-xylene is the other name for o-dimethylbenzene. Therefore, we can draw its structure as-

Now, let us see the mechanism of ozonolysis of the above compound.

Now, if the bond cleaving was different and included the two methyl groups in two different five-membered rings instead of the same ring as above then, we would receive a mixture of products which contained methylglyoxal and glyoxal. We can draw it as-

We can understand from the above discussion that o-xylene will give a mixture of glyoxal, methylglyoxal, and dimethyl glyoxal since there are two resonating structures of xylene.
These products suggest that benzene has 3 double bonds in the ring and their positions are as shown. (These resonant structures are obtained from two Kekule structures).
This is also a test to prove that benzene (in this case o-xylene) is a hybrid of two Kekule structures.
So, the correct answer is “Option D”.
Note: Here we saw that 1,2-dimethylbenzene (o-xylene) on ozonolysis gave glyoxal, methylglyoxal, and dimethyl glyoxal in 3:2:1 molar ratio. This proves the existence of two resonance structures for benzene and its derivatives.
Kekule structure: A Lewis structure in which bonded electron pairs in covalent bonds are shown as lines. The most famous Kekule structures are what we would now call the two most significant resonance contributors of benzene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




