
P – Chloroaniline and anilinium hydrochloride can be distinguished by:
A. Sandmeyer’s Reaction
B. \[NaHC{O_3}\]
C. \[AgN{O_3}\]
D. Carbylamine test
Answer
578.4k+ views
Hint: To solve this question, we need to first understand the nature and properties of the compounds given to us, i.e. P – Chloroaniline and anilinium hydroxide. We also need to understand the different processes given in the options, and their reactions with the given compounds.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
P – Chloroaniline can be explained as an aniline molecule (benzene molecule with an amine functional group), which has a chlorine atom at its para position, or in simpler terms, at the position 04 carbon atom in the benzene ring. P – Chloroaniline can be found as either a white or a pale yellow solid. The molecular structure for the same can be given as:
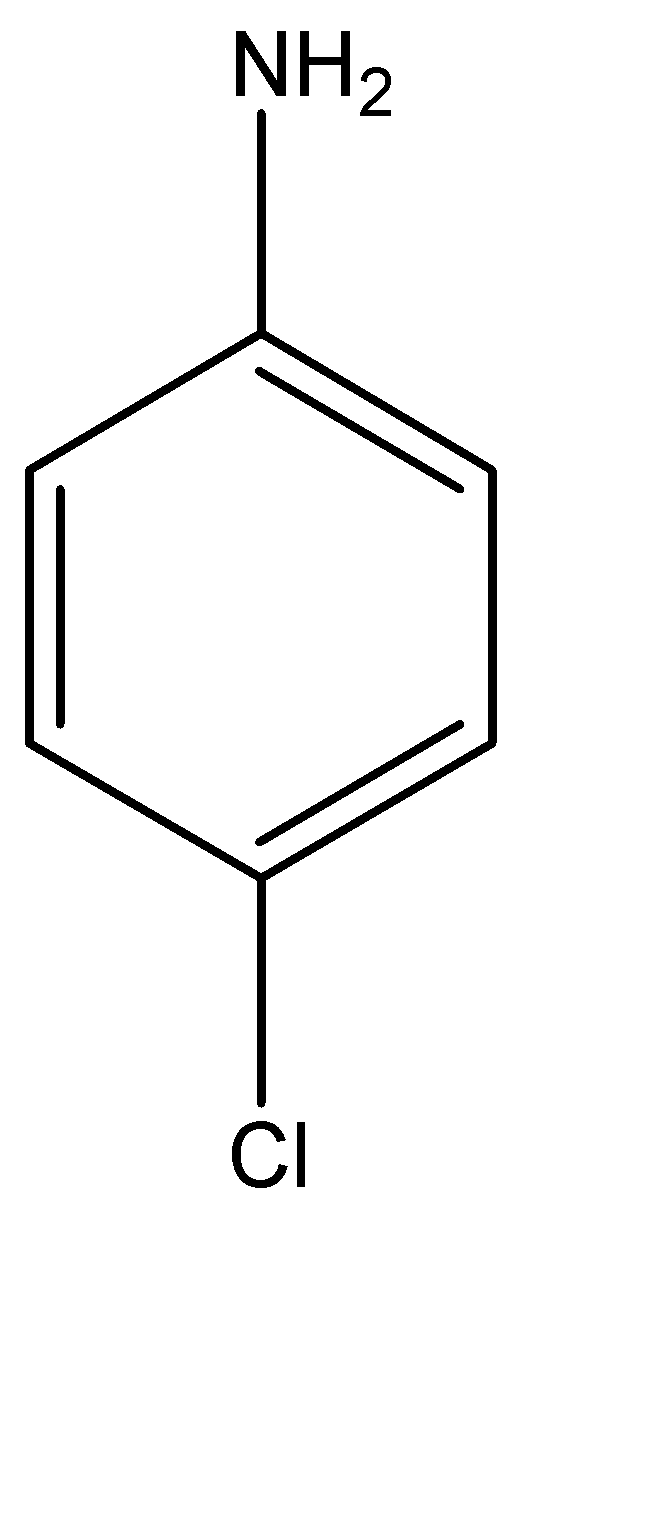
Anilinium hydroxide can be explained as a hydroxy substituted anilinium ion. It is a little difficult to explain the structure of anilinium ion verbally, hence, the molecular structure can be given as:
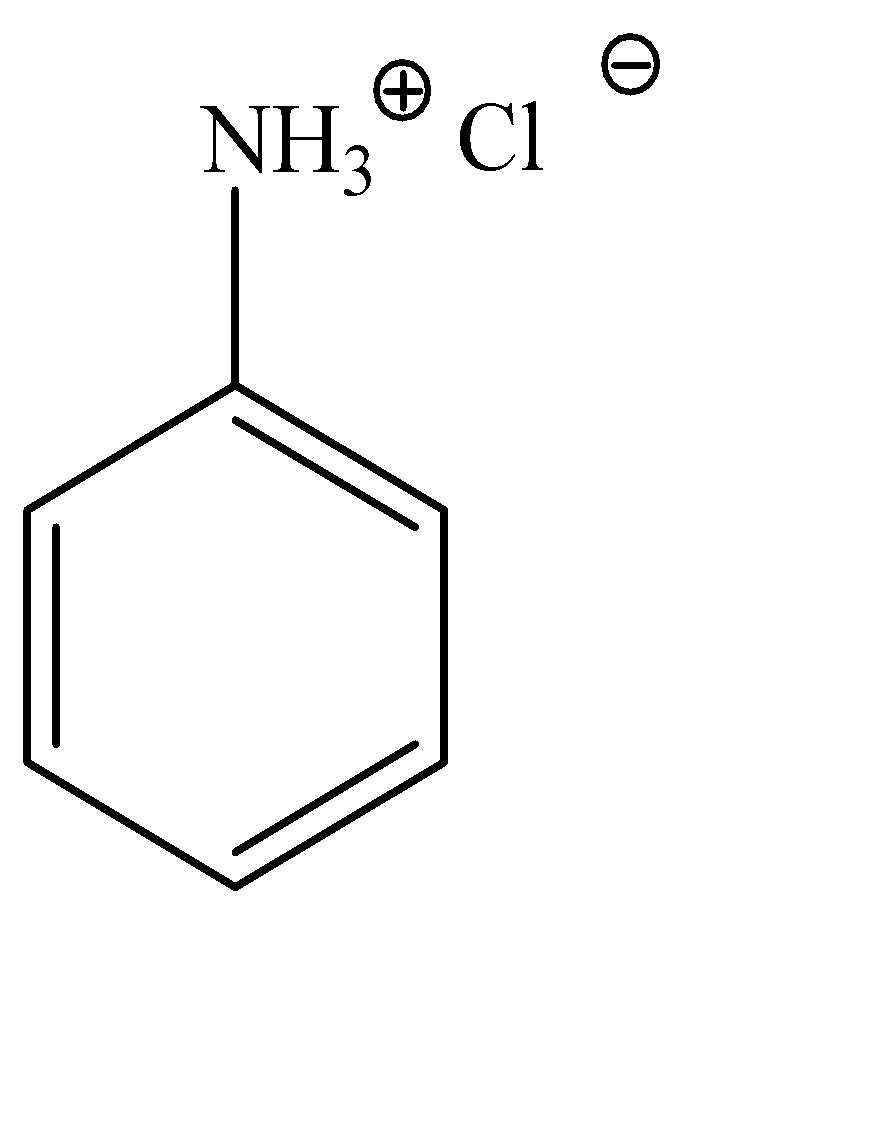
Now, we can observe that both these compounds have the aniline molecule in common, but the position of the chloride ion is varied. Hence, we would need a test that would identify the difference in the nature of both these chloride ions.
Now, let us discuss the options given to us:
1.Sandmeyer test: Sandmeyer’s test is useful for differentiating aryl diazonium salts from aryl halides. In our case, we have an aryl halide, but the other compound is not a diazonium salt. Hence, we cannot use this test.
2.Carbylamine test: Carbylamine test is used to detect the presence of primary amines. Now, both the compounds given to us are primary amines. Hence, both of them would yield a positive result.
3. \[AgN{O_3}\]: When anilinium hydrochloride is reacted with \[AgN{O_3}\], then it results in the formation of AgCl. AgCl is formed as a white precipitate and hence is easily distinguishable. This reaction is not shown by P – chloroaniline.
Hence, Option C is the correct option.
Note: p-Chloro aniline is used in the industrial production of pesticides, drugs, and dyestuffs. It is a precursor to the widely used antimicrobial and bactericide chlorhexidine
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
P – Chloroaniline can be explained as an aniline molecule (benzene molecule with an amine functional group), which has a chlorine atom at its para position, or in simpler terms, at the position 04 carbon atom in the benzene ring. P – Chloroaniline can be found as either a white or a pale yellow solid. The molecular structure for the same can be given as:

Anilinium hydroxide can be explained as a hydroxy substituted anilinium ion. It is a little difficult to explain the structure of anilinium ion verbally, hence, the molecular structure can be given as:

Now, we can observe that both these compounds have the aniline molecule in common, but the position of the chloride ion is varied. Hence, we would need a test that would identify the difference in the nature of both these chloride ions.
Now, let us discuss the options given to us:
1.Sandmeyer test: Sandmeyer’s test is useful for differentiating aryl diazonium salts from aryl halides. In our case, we have an aryl halide, but the other compound is not a diazonium salt. Hence, we cannot use this test.
2.Carbylamine test: Carbylamine test is used to detect the presence of primary amines. Now, both the compounds given to us are primary amines. Hence, both of them would yield a positive result.
3. \[AgN{O_3}\]: When anilinium hydrochloride is reacted with \[AgN{O_3}\], then it results in the formation of AgCl. AgCl is formed as a white precipitate and hence is easily distinguishable. This reaction is not shown by P – chloroaniline.
Hence, Option C is the correct option.
Note: p-Chloro aniline is used in the industrial production of pesticides, drugs, and dyestuffs. It is a precursor to the widely used antimicrobial and bactericide chlorhexidine
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




