
How many \[P - H\] bonds are present in \[{H_3}P{O_2}\] ?
Answer
583.2k+ views
Hint: \[{H_3}P{O_2}\] is formed by keeping phosphorous as the central atom. The atomic number of phosphorus is 15 and hence its electronic configuration is given as: \[1{s^2}2{s^2}2{p^6}3{s^5}\] . Hence, there are 5 electrons present in the valence shell. Now phosphorus forms bonds using all 5 of these electrons while forming \[{H_3}P{O_2}\] .
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Oxoacids are those acids which contain an oxygen atom in them. Some elements form multiple oxoacids like phosphorus and sulphur. This is possible because of the electronic configuration of these elements.
Phosphorus forms different oxoacids like orthophosphoric acid, pyrophosphoric acid, ortho phosphoric acid and hypo phosphorous acid. All these acids have the same constituent elements: phosphorus, hydrogen and oxygen. These elements are used in different quantities, bonding between different atoms and molecule positions to form their corresponding compounds.
The chemical formula of the oxoacids of phosphorus are: orthophosphoric acid \[({H_3}P{O_4})\] , pyrophosphoric acid \[({H_4}{P_2}{O_7})\] , ortho phosphoric acid \[({H_3}P{O_3})\] and hypo phosphorous acid \[({H_3}P{O_2})\] . From this data, we can say that the compound given to us is hypo phosphorous acid.
The central atom forms a double bond with one oxygen atom present in the structure. Out of the three hydrogen atoms that remain, 2 of them are all individually bonded to the central atom. The remaining one oxygen and one hydrogen atom form a hydroxyl group \[\left( { - OH} \right)\] and gets attached directly to the central atom via a single sigma bond. Hence, the molecular structure of \[{H_3}P{O_2}\] can be given as:
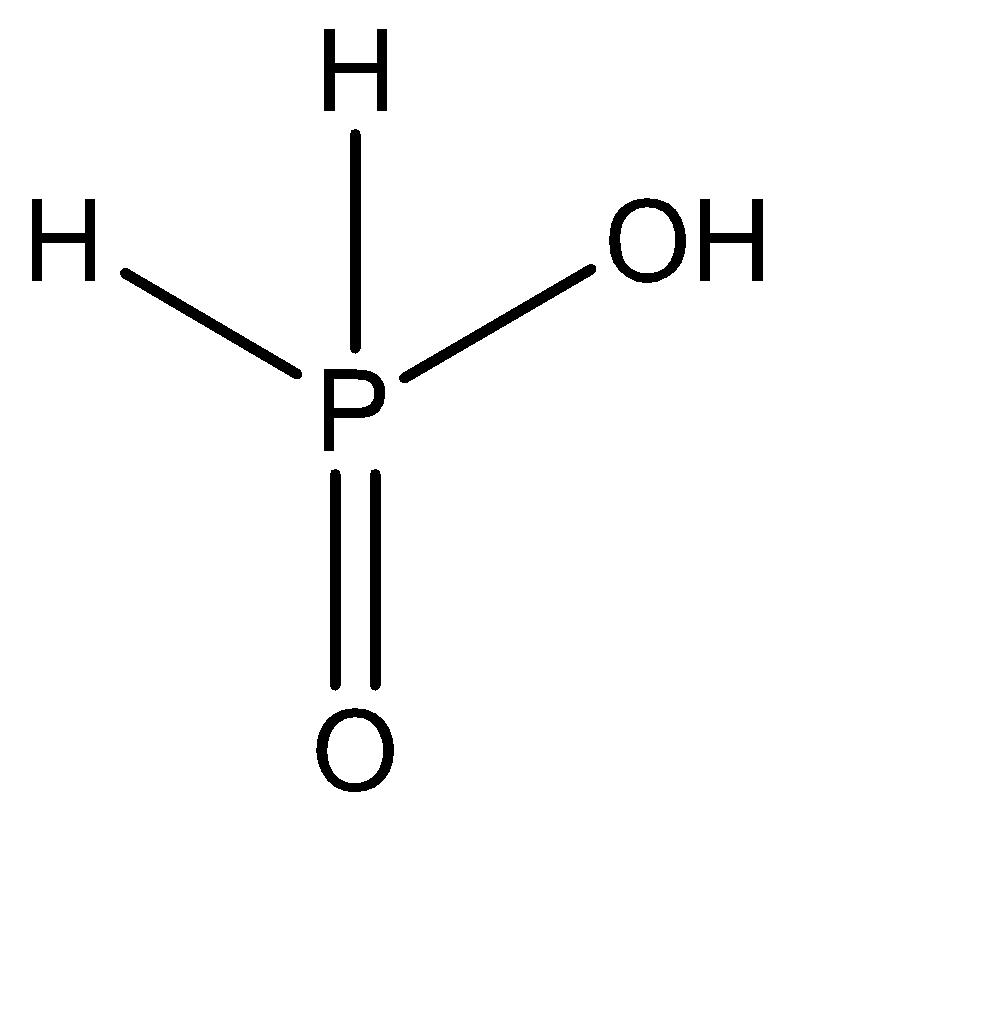
Hence, there are 2 \[P - H\] bonds present in this compound.
Note: The 2 hydrogen bonds that are bonded to the central atom can dissociate from the main molecule. This means that hypo phosphorous acid has the capacity to release 2 hydrogen atoms, which makes it a dibasic acid. The third hydrogen atom is not released because it is linked with an oxygen atom.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Oxoacids are those acids which contain an oxygen atom in them. Some elements form multiple oxoacids like phosphorus and sulphur. This is possible because of the electronic configuration of these elements.
Phosphorus forms different oxoacids like orthophosphoric acid, pyrophosphoric acid, ortho phosphoric acid and hypo phosphorous acid. All these acids have the same constituent elements: phosphorus, hydrogen and oxygen. These elements are used in different quantities, bonding between different atoms and molecule positions to form their corresponding compounds.
The chemical formula of the oxoacids of phosphorus are: orthophosphoric acid \[({H_3}P{O_4})\] , pyrophosphoric acid \[({H_4}{P_2}{O_7})\] , ortho phosphoric acid \[({H_3}P{O_3})\] and hypo phosphorous acid \[({H_3}P{O_2})\] . From this data, we can say that the compound given to us is hypo phosphorous acid.
The central atom forms a double bond with one oxygen atom present in the structure. Out of the three hydrogen atoms that remain, 2 of them are all individually bonded to the central atom. The remaining one oxygen and one hydrogen atom form a hydroxyl group \[\left( { - OH} \right)\] and gets attached directly to the central atom via a single sigma bond. Hence, the molecular structure of \[{H_3}P{O_2}\] can be given as:

Hence, there are 2 \[P - H\] bonds present in this compound.
Note: The 2 hydrogen bonds that are bonded to the central atom can dissociate from the main molecule. This means that hypo phosphorous acid has the capacity to release 2 hydrogen atoms, which makes it a dibasic acid. The third hydrogen atom is not released because it is linked with an oxygen atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




