
How many $P - O - P$ bonds(s) is/are present in ${H_6}{P_6}{O_{18}}$ ?
Answer
582.9k+ views
Hint: ${H_6}{P_6}{O_{18}}$ is known as meta-phosphoric acid. Generally, the number of ${\text{P - O - P}}$ bonds in cyclic metaphosphoric acid is three. ${\text{P - O - P}}$ bonds are the bond linkages present between two phosphorus atoms and one oxygen atom.
Complete step by step answer: The general formula of metaphosphoric acids is ${\text{(HP}}{{\text{O}}_{\text{3}}}{\text{)n}}$ where n denotes the number of phosphoric acid units present in the ring. ‘n’ can be equal to three or greater than three. Metaphosphoric acids have phosphorus in the oxidation state of ${\text{ + 5}}$. It generally decomposes in water and is soluble in alcohol. It is an odorless, glassy substance.
${H_6}{P_6}{O_{18}}$ is meta-phosphoric acid . it is a corrosive inorganic , cyclic polyphosphate formed from bonded phosphoric acid units.
It has applications in biochemistry, agriculture , pharmacy and chemical research.
Structure of meta-phosphoric acid is :
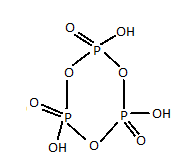
As it is clear from the above representation of meta-phosphoric acid, there are three $P - O - P$ bonds.
So, the answer to the question will be three.
Additional Information:
-Meta-phosphoric acid is used by food analytics to determine the vitamins.
-The biochemistry also makes use of this compound to precipitate protein in biological liquids such as blood and urine.
-For vets, it can be used to check the urea in cattle blood.
-It can also be used as an additive in the production of diagnostic test strips for the pharmaceutical industry.
-Metaphosphoric acid is used to prevent oxidation of reduced glutathione.
Note: Meta-phosphoric acid or ${H_6}{P_6}{O_{18}}$ has various uses in various fields. The number of $P - O - P$ bonds in cyclic meta-phosphoric acid is three. Moreover, metaphosphoric acid seems to have some hazardous effects, it can cause burns and may cause injuries to the upper respiratory tract and lungs if inhaled.
Complete step by step answer: The general formula of metaphosphoric acids is ${\text{(HP}}{{\text{O}}_{\text{3}}}{\text{)n}}$ where n denotes the number of phosphoric acid units present in the ring. ‘n’ can be equal to three or greater than three. Metaphosphoric acids have phosphorus in the oxidation state of ${\text{ + 5}}$. It generally decomposes in water and is soluble in alcohol. It is an odorless, glassy substance.
${H_6}{P_6}{O_{18}}$ is meta-phosphoric acid . it is a corrosive inorganic , cyclic polyphosphate formed from bonded phosphoric acid units.
It has applications in biochemistry, agriculture , pharmacy and chemical research.
Structure of meta-phosphoric acid is :

As it is clear from the above representation of meta-phosphoric acid, there are three $P - O - P$ bonds.
So, the answer to the question will be three.
Additional Information:
-Meta-phosphoric acid is used by food analytics to determine the vitamins.
-The biochemistry also makes use of this compound to precipitate protein in biological liquids such as blood and urine.
-For vets, it can be used to check the urea in cattle blood.
-It can also be used as an additive in the production of diagnostic test strips for the pharmaceutical industry.
-Metaphosphoric acid is used to prevent oxidation of reduced glutathione.
Note: Meta-phosphoric acid or ${H_6}{P_6}{O_{18}}$ has various uses in various fields. The number of $P - O - P$ bonds in cyclic meta-phosphoric acid is three. Moreover, metaphosphoric acid seems to have some hazardous effects, it can cause burns and may cause injuries to the upper respiratory tract and lungs if inhaled.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




