
How many pairs of electrons do the two oxygen atoms in an oxygen molecule share with each other?
Answer
531.9k+ views
Hint: When a pair of electrons are shared by two atoms, a covalent bond is formed. So to determine the pair of electrons shared by the two oxygen atoms, we need to find the bonding in the oxygen molecule.
Complete answer:
To determine the bonding in an oxygen molecule, we first need to know its molecular formula. The molecular formula of oxygen molecules is ${{O}_{2}}$.
Now, the chemical bonding in a molecule can be depicted using the Lewis dot structures.
To draw the Lewis dot structure of oxygen molecule,
- We first need to know the electronic configuration of oxygen atoms.
\[^{8}O=1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- Determine the number of valence electrons.
Oxygen atom has 6 electrons in its outermost (n = 2) shell and hence it has 6 valence electrons.
The valence electrons are the ones that participate in molecule or chemical bond formation.
- Arrange the valence electrons around the oxygen atoms.
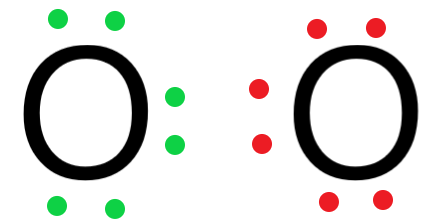
- Since the oxygen atom has 6 valence electrons, it needs 2 electrons to completely fill its outermost shell and satisfy the octet rule.
The two electrons required by the oxygen atom can be obtained by the other oxygen molecule by sharing.
Each oxygen atom will share two electrons with the other oxygen atom and form two covalent bonds or a double bond.
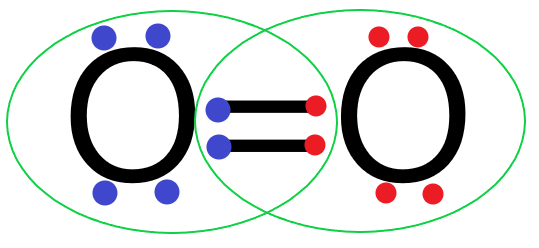
So we can see that the two oxygen atoms in an oxygen molecule share 2 pairs of electrons with each other
Note:
When there exists a charge atom, electrons equal to the magnitude of the charge are added (in case of a negatively charged anion) or subtracted (in case of a positive charged cation) from the Lewis dot diagram.
Complete answer:
To determine the bonding in an oxygen molecule, we first need to know its molecular formula. The molecular formula of oxygen molecules is ${{O}_{2}}$.
Now, the chemical bonding in a molecule can be depicted using the Lewis dot structures.
To draw the Lewis dot structure of oxygen molecule,
- We first need to know the electronic configuration of oxygen atoms.
\[^{8}O=1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- Determine the number of valence electrons.
Oxygen atom has 6 electrons in its outermost (n = 2) shell and hence it has 6 valence electrons.
The valence electrons are the ones that participate in molecule or chemical bond formation.
- Arrange the valence electrons around the oxygen atoms.

- Since the oxygen atom has 6 valence electrons, it needs 2 electrons to completely fill its outermost shell and satisfy the octet rule.
The two electrons required by the oxygen atom can be obtained by the other oxygen molecule by sharing.
Each oxygen atom will share two electrons with the other oxygen atom and form two covalent bonds or a double bond.

So we can see that the two oxygen atoms in an oxygen molecule share 2 pairs of electrons with each other
Note:
When there exists a charge atom, electrons equal to the magnitude of the charge are added (in case of a negatively charged anion) or subtracted (in case of a positive charged cation) from the Lewis dot diagram.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




