
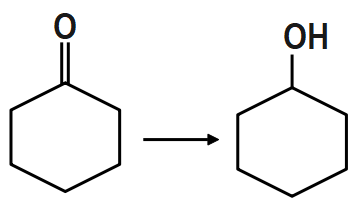
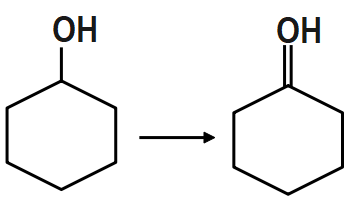
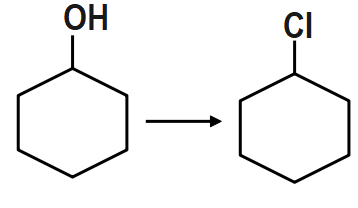
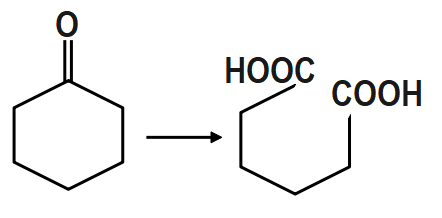
PCC (Pyridinium Chlorochromate) is a good reagent for which of the following transformations?
(A)
(B)
(C)
(D)




Answer
529.2k+ views
Hint :We know that the PCC stands for pyridinium chlorochromate. Pyridinium chlorochromate is formed by the reaction of pyridine and chromium oxide, hydrochloric acid. You can now protonate the nitrogen atom present on the pyridine molecule and then perform the reaction. Based on this you can determine the possible structure of PCC i.e. pyridinium chlorochromate.
Complete Step By Step Answer:
By knowing the chemical name of PCC, the structure of PCC can be identified. PCC is an organic compound known to be Pyridinium Chlorochromate. From the name we can say there is a pyridine molecule having chlorine and chromate ions.
Pyridinium chlorochromate (PCC) consists of a pyridinium cation, $ {{[{{C}_{5}}{{H}_{5}}NH]}^{+}} $ and a tetrahedral chlorochromate anion. It is a yellow-orange salt, used as an oxidant. It is commercially available. This reagent was discovered by accident which was originally prepared via addition of pyridine into a cold solution of chromium trioxide in concentrated hydrochloric acid. You can see the equation that represents the chemical reaction of Pyridinium chlorochromate formation
PCC is the most suitable reagent to oxidize primary alcohol to aldehydes and secondary alcohols to ketones without affecting any other functional group. Thus it offers the advantage of selective oxidation. In fact it is more selective than the related Jones reagent, so there is little chance of over-oxidation to form carboxylic acids as long as water is not present in the reaction mixture. It also converts suitable unsaturated alcohols and aldehydes to cyclohexane. One disadvantage to the use of PCC is its toxicity. Other more convenient or less toxic reagents for oxidizing alcohols is the dimethyl sulfoxide. PCC is soluble in solvents like acetone, acetonitrile.
Therefore, option A is the correct answer.
Note :
Remember that generally the confusion may arise to choose in between option a and c. Pyridinium chlorochromate is formed by combining the compounds, pyridinium cation and a chlorochromate anion. We have to choose the option resembling these.
Complete Step By Step Answer:
By knowing the chemical name of PCC, the structure of PCC can be identified. PCC is an organic compound known to be Pyridinium Chlorochromate. From the name we can say there is a pyridine molecule having chlorine and chromate ions.
Pyridinium chlorochromate (PCC) consists of a pyridinium cation, $ {{[{{C}_{5}}{{H}_{5}}NH]}^{+}} $ and a tetrahedral chlorochromate anion. It is a yellow-orange salt, used as an oxidant. It is commercially available. This reagent was discovered by accident which was originally prepared via addition of pyridine into a cold solution of chromium trioxide in concentrated hydrochloric acid. You can see the equation that represents the chemical reaction of Pyridinium chlorochromate formation
PCC is the most suitable reagent to oxidize primary alcohol to aldehydes and secondary alcohols to ketones without affecting any other functional group. Thus it offers the advantage of selective oxidation. In fact it is more selective than the related Jones reagent, so there is little chance of over-oxidation to form carboxylic acids as long as water is not present in the reaction mixture. It also converts suitable unsaturated alcohols and aldehydes to cyclohexane. One disadvantage to the use of PCC is its toxicity. Other more convenient or less toxic reagents for oxidizing alcohols is the dimethyl sulfoxide. PCC is soluble in solvents like acetone, acetonitrile.
Therefore, option A is the correct answer.
Note :
Remember that generally the confusion may arise to choose in between option a and c. Pyridinium chlorochromate is formed by combining the compounds, pyridinium cation and a chlorochromate anion. We have to choose the option resembling these.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




