
$\text{PC}{{\text{l}}_{5}}$ has a shape of trigonal bipyramidal whereas $\text{I}{{\text{F}}_{5}}$ has the shape of a square pyramid. It is due to
A. presence of an unshared electron pair on I which is oriented so as to minimise the repulsion while in P in $\text{PC}{{\text{l}}_{5}}$ has no unshared pair.
B. octet of P is complete while that of I is incomplete.
C. P and I are of different groups.
D. F and Cl have different degrees of repulsion.
Answer
529.7k+ views
Hint: Phosphorus has five electrons in the outermost shell and it can make five bonds with other molecules whereas iodine has 7 electrons and has a tendency to accept a pair of electrons so it forms mainly a single bond.
Complete answer:
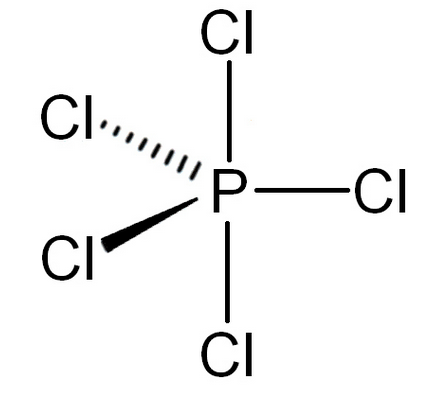
-In $\text{PC}{{\text{l}}_{5}}$, the electronic configuration of phosphorus is $\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{6}}\text{ 3}{{\text{s}}^{2}}\text{ 3}{{\text{p}}^{3}}$ to form five bonds with chlorine electron form s-orbital is excited to the vacant d-orbital due to which phosphorus gains the ability to make 5 bonds.
-Now, it has a total of five unpaired electrons.
-So, the hybridisation of phosphorus is $\text{s}{{\text{p}}^{3}}\text{d}$ and the geometry will be trigonal bipyramidal.
-Because it has 5 bond pairs and 0 lone pairs.
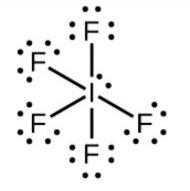
-In $\text{I}{{\text{F}}_{5}}$, according to the electronic configuration of iodine two electrons from 5p-orbital are excited to move to the 5d-orbital.
-So, now iodine tends to make 5 bonds and along with it have one lone pair.
-The lone pair will cause the repulsion towards the bond pair and increases the bond angles that’s why it is oriented in such a way that it causes less repulsion.
-So, the hybridisation will be $\text{s}{{\text{p}}^{3}}{{\text{d}}^{2}}$and the geometry will be square pyramidal.
-The structure is:
So, the correct answer is “Option A”.
Note: The lone pair- lone pair repulsion causes maximum repulsion than lone pair-bond pair and lone pair-bond pair repulsion is more than the bond pair-bond pair repulsion.
Complete answer:
-In $\text{PC}{{\text{l}}_{5}}$, the electronic configuration of phosphorus is $\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{6}}\text{ 3}{{\text{s}}^{2}}\text{ 3}{{\text{p}}^{3}}$ to form five bonds with chlorine electron form s-orbital is excited to the vacant d-orbital due to which phosphorus gains the ability to make 5 bonds.
-Now, it has a total of five unpaired electrons.
-So, the hybridisation of phosphorus is $\text{s}{{\text{p}}^{3}}\text{d}$ and the geometry will be trigonal bipyramidal.
-Because it has 5 bond pairs and 0 lone pairs.

-In $\text{I}{{\text{F}}_{5}}$, according to the electronic configuration of iodine two electrons from 5p-orbital are excited to move to the 5d-orbital.
-So, now iodine tends to make 5 bonds and along with it have one lone pair.
-The lone pair will cause the repulsion towards the bond pair and increases the bond angles that’s why it is oriented in such a way that it causes less repulsion.
-So, the hybridisation will be $\text{s}{{\text{p}}^{3}}{{\text{d}}^{2}}$and the geometry will be square pyramidal.
-The structure is:

So, the correct answer is “Option A”.
Note: The lone pair- lone pair repulsion causes maximum repulsion than lone pair-bond pair and lone pair-bond pair repulsion is more than the bond pair-bond pair repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




