
Phenol and ethanol are distinguished by the reaction with:
A) Red litmus paper
B) \[{\text{NaHC}}{{\text{O}}_{\text{3}}}\]
C) \[{\text{FeC}}{{\text{l}}_{\text{3}}}\]
D) \[{\text{NaOH}}\]
Answer
523.2k+ views
Hint:Determine whether phenol and ethanol are acidic, basic or neutral in nature. Red litmus paper turns to blue in the basic medium. In presence of acid \[{\text{NaHC}}{{\text{O}}_{\text{3}}}\] dissociate and liberate \[{\text{C}}{{\text{O}}_{\text{2}}}\]gas. Write the reaction of phenol and ethanol with \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] and \[{\text{NaOH}}\] and determine if there is any colour change.
Complete step-by-step answer:
The structure of phenol and ethanol are as follows:
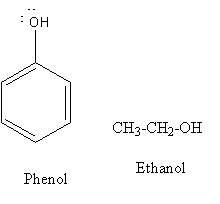
Now using chemical properties of phenol and alcohol we will decide the distinguishing test for phenol and ethanol.
A) Red litmus paper : Litmus paper is used to distinguish acid from the base. Red litmus paper turns to blue in basic medium while remaining unchanged in acidic medium. Phenol is acidic in nature while ethanol is a very weak acid. It is almost neutral. So red litmus paper will remain unchanged in both phenol and ethanol so it cannot be used to distinguish Phenol and ethanol. Thus, option (A) is an incorrect answer.
B)\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\] : Sodium bicarbonate (\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\]) is a weak base in presence of acid it dissociates into \[{\text{C}}{{\text{O}}_{\text{2}}}\]gas and water.
\[{\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{ + H + }} \to {\text{C}}{{\text{O}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
Though phenol is acidic in nature it is very weak acid so it will not react with Sodium bicarbonate,\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\].
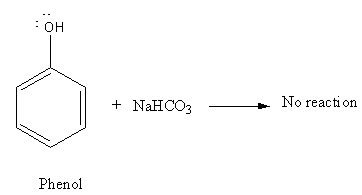
As ethanol is neutral in nature it will not react with Sodium bicarbonate,\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\].
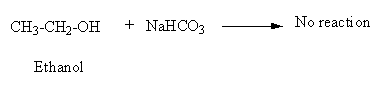
So, there will be no reaction of \[{\text{NaHC}}{{\text{O}}_{\text{3}}}\] with phenol and ethanol. Thus, option (B) is an incorrect answer.
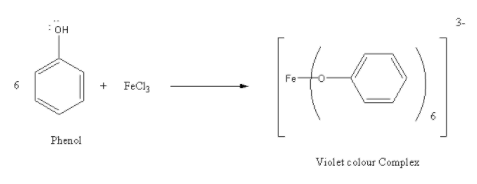
C) \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] : Addition of \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] in phenol gives a violet colour complex. While addition \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] to phenol does not show any colour change. So, \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] is used to distinguish phenol from ethanol. Thus, the correct option is (C) \[{\text{FeC}}{{\text{l}}_{\text{3}}}\].
D) \[{\text{NaOH}}\] : Sodium hydroxide,\[{\text{NaOH}}\] is a strong base so it reacts with acidic phenol and gives phenoxide. However, since ethanol is neutral in nature it does not react with \[{\text{NaOH}}\].
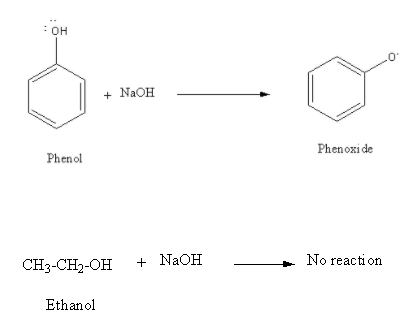
Though phenol reacts with \[{\text{NaOH}}\] but it does not show any colour change so \[{\text{NaOH}}\] can not be used to distinguish phenol from ethanol. Thus, option (D) is an incorrect answer.
Hence the correct answer is option ‘C’.
Note:To distinguish two different colourless species we have to carry out chemical reactions. We have to select the reagent that shows colour change after reacting with one species and remain unchanged after addition in another species. Litmus test can be used only if one species is acidic in nature and another species is basic in nature.
Complete step-by-step answer:
The structure of phenol and ethanol are as follows:

Now using chemical properties of phenol and alcohol we will decide the distinguishing test for phenol and ethanol.
A) Red litmus paper : Litmus paper is used to distinguish acid from the base. Red litmus paper turns to blue in basic medium while remaining unchanged in acidic medium. Phenol is acidic in nature while ethanol is a very weak acid. It is almost neutral. So red litmus paper will remain unchanged in both phenol and ethanol so it cannot be used to distinguish Phenol and ethanol. Thus, option (A) is an incorrect answer.
B)\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\] : Sodium bicarbonate (\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\]) is a weak base in presence of acid it dissociates into \[{\text{C}}{{\text{O}}_{\text{2}}}\]gas and water.
\[{\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{ + H + }} \to {\text{C}}{{\text{O}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
Though phenol is acidic in nature it is very weak acid so it will not react with Sodium bicarbonate,\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\].

As ethanol is neutral in nature it will not react with Sodium bicarbonate,\[{\text{NaHC}}{{\text{O}}_{\text{3}}}\].
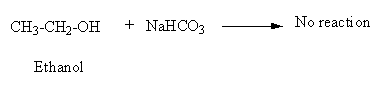
So, there will be no reaction of \[{\text{NaHC}}{{\text{O}}_{\text{3}}}\] with phenol and ethanol. Thus, option (B) is an incorrect answer.
C) \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] : Addition of \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] in phenol gives a violet colour complex. While addition \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] to phenol does not show any colour change. So, \[{\text{FeC}}{{\text{l}}_{\text{3}}}\] is used to distinguish phenol from ethanol. Thus, the correct option is (C) \[{\text{FeC}}{{\text{l}}_{\text{3}}}\].

D) \[{\text{NaOH}}\] : Sodium hydroxide,\[{\text{NaOH}}\] is a strong base so it reacts with acidic phenol and gives phenoxide. However, since ethanol is neutral in nature it does not react with \[{\text{NaOH}}\].

Though phenol reacts with \[{\text{NaOH}}\] but it does not show any colour change so \[{\text{NaOH}}\] can not be used to distinguish phenol from ethanol. Thus, option (D) is an incorrect answer.
Hence the correct answer is option ‘C’.
Note:To distinguish two different colourless species we have to carry out chemical reactions. We have to select the reagent that shows colour change after reacting with one species and remain unchanged after addition in another species. Litmus test can be used only if one species is acidic in nature and another species is basic in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




