
Phenol is an organic compound even though it is soluble in water due to the presence of:
(a)- Ionic bonding
(b)- Covalent bonding
(c)- Hydrogen bonding
(d)- Coordinate bonding
Answer
531.6k+ views
Hint: Phenol is an organic compound that is an aromatic compound having a benzene ring and on the benzene ring there is a hydroxyl group present. So, there is an electronegative atom in the phenol, i.e., oxygen atom which will form bonding with the hydrogen atom in water.
Complete answer:
Phenol is an organic compound that is an aromatic compound having a benzene ring and on the benzene ring, there is a hydroxyl group present. Its formula is ${{C}_{6}}{{H}_{5}}OH$, and its formula is given below:
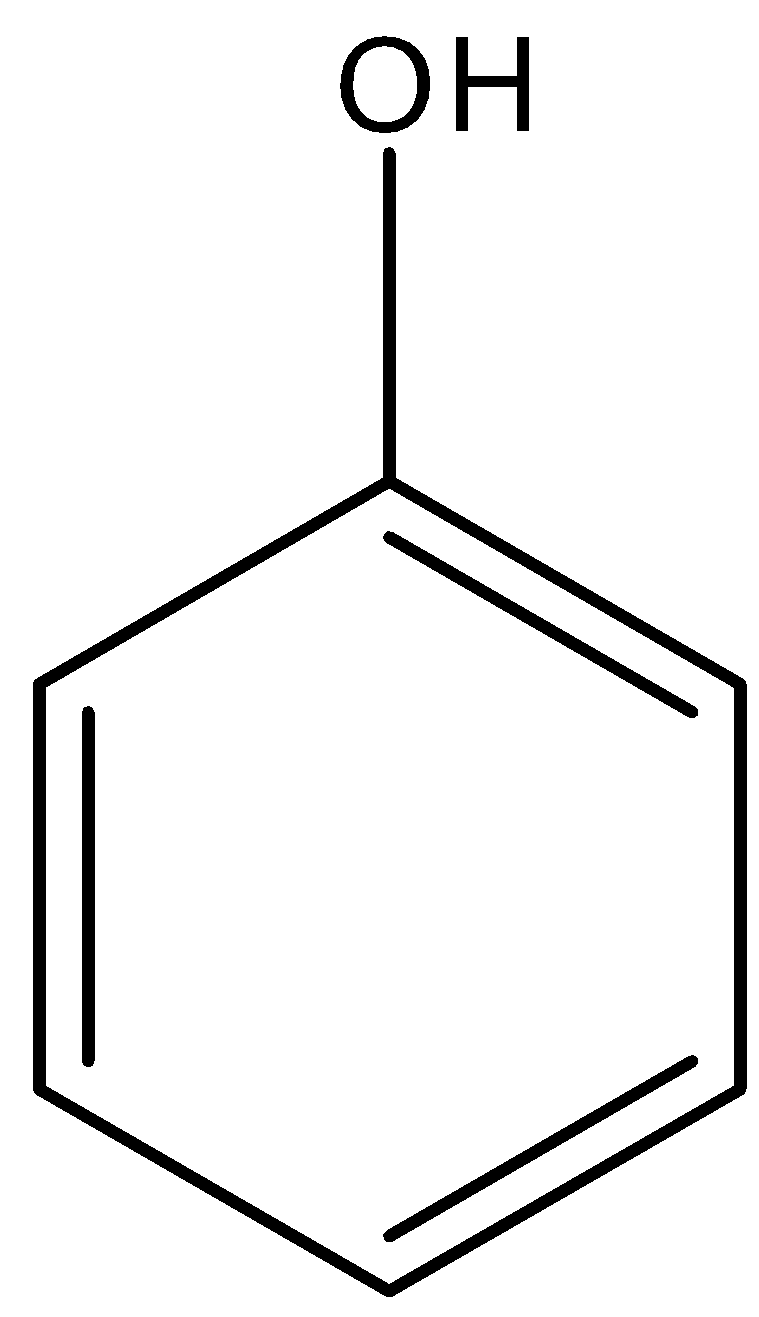
So, there is an electronegative atom in the phenol, i.e., oxygen atom. And we know that most three electronegative atoms in the periodic table, i.e., nitrogen, oxygen, and fluorine atoms have a special ability to form bonding with the hydrogen atom, and this bonding is known as hydrogen bonding.
So, when the phenol is dissolved in water the oxygen atom of the phenol atom will form hydrogen bonding with hydrogen atoms in the water. Therefore, due to this hydrogen bonding, phenol is soluble in water as hydrogen bonding is a strong bonding.
Therefore, the correct answer is an option (c)- Hydrogen bonding.
Note:
Only nitrogen, oxygen, and fluorine can form the hydrogen bonding, and due to this bonding the solubility increases, boiling point increases, etc. This bonding is stronger than covalent bonding.
Complete answer:
Phenol is an organic compound that is an aromatic compound having a benzene ring and on the benzene ring, there is a hydroxyl group present. Its formula is ${{C}_{6}}{{H}_{5}}OH$, and its formula is given below:

So, there is an electronegative atom in the phenol, i.e., oxygen atom. And we know that most three electronegative atoms in the periodic table, i.e., nitrogen, oxygen, and fluorine atoms have a special ability to form bonding with the hydrogen atom, and this bonding is known as hydrogen bonding.
So, when the phenol is dissolved in water the oxygen atom of the phenol atom will form hydrogen bonding with hydrogen atoms in the water. Therefore, due to this hydrogen bonding, phenol is soluble in water as hydrogen bonding is a strong bonding.
Therefore, the correct answer is an option (c)- Hydrogen bonding.
Note:
Only nitrogen, oxygen, and fluorine can form the hydrogen bonding, and due to this bonding the solubility increases, boiling point increases, etc. This bonding is stronger than covalent bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




