
When phenol is treated with excess bromine water, it gives:
A.m-bromophenol
B.o- and p- bromophenol
C.2,4-dibromophenol
D.2,4,6-tribromophenol
Answer
530.5k+ views
Hint:
Phenol is an organic compound that has an alcohol group attached to the benzene ring. Adding excess bromine water to the compound will result in Bromination and will give a white precipitate.
Complete step by step answer:
Phenol is an aromatic hydrocarbon, composed of benzene which has a hydroxyl group attached to it.
The alcohol group (-OH) in phenol is an activating group, i.e. it activates the ring by releasing electrons. Because of activation, the ring becomes very reactive. It is therefore, an ortho – para directing group. This is also known as 2,4-directing effect.
Bromination of phenol by excess bromine water will result in multiple substitution around the ring. This reaction is so feasible that it can take place in cold solvent in the absence of any catalyst.
The reaction can be represented as –
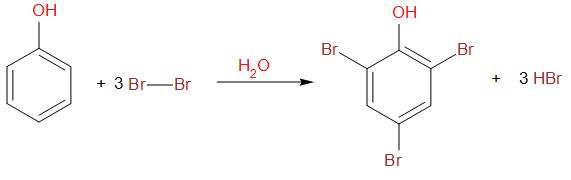
Therefore, the answer is – option (d) – When phenol is treated with excess bromine water, it gives 2,4,6-tribromophenol.
Additional Information: Bromine water is an indication test for phenol because the compound 2,4,6-tribromophenol is a white precipitate with an antiseptic smell.
Note:
If Bromination is done using a less polar solvent such as carbon disulfide, carbon tetrachloride, etc., it will give a monosubstituted compound – p-bromophenol, o-bromophenol is the minor product.
Phenol is an organic compound that has an alcohol group attached to the benzene ring. Adding excess bromine water to the compound will result in Bromination and will give a white precipitate.
Complete step by step answer:
Phenol is an aromatic hydrocarbon, composed of benzene which has a hydroxyl group attached to it.
The alcohol group (-OH) in phenol is an activating group, i.e. it activates the ring by releasing electrons. Because of activation, the ring becomes very reactive. It is therefore, an ortho – para directing group. This is also known as 2,4-directing effect.
Bromination of phenol by excess bromine water will result in multiple substitution around the ring. This reaction is so feasible that it can take place in cold solvent in the absence of any catalyst.
The reaction can be represented as –

Therefore, the answer is – option (d) – When phenol is treated with excess bromine water, it gives 2,4,6-tribromophenol.
Additional Information: Bromine water is an indication test for phenol because the compound 2,4,6-tribromophenol is a white precipitate with an antiseptic smell.
Note:
If Bromination is done using a less polar solvent such as carbon disulfide, carbon tetrachloride, etc., it will give a monosubstituted compound – p-bromophenol, o-bromophenol is the minor product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




