
Phenol reacts with acetone in the presence of conc. sulphuric acid to form a $ {C_{15}}{H_{16}}{O_2} $ product. Which of the following compounds is this product?
(A)
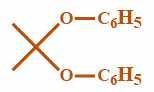
(B)
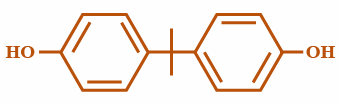
©
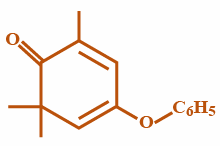
(D)
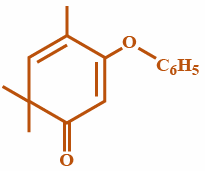




Answer
544.5k+ views
Hint :To solve this question, we must first understand the whole concept of Phenol and its reactions. Then we need to use the correct reaction mechanism and then only we can have our correct answer.
Complete Step By Step Answer:
Before jumping to the solution, let’s recall the basic concepts about Phenol and its reaction:
As we know that, Phenol consists of a hydroxyl group and phenyl group attached to each other. It dissolves in water. Earlier it was used as carbolic soap. It is mildly acidic and is corrosive to the respiratory tract, eyes, and skin.
Phenol is a crystalline solid white in color and needs to be handled with care as it can cause chemical burns. It is also known as phenolic acid. If a compound consists of a six-membered aromatic ring and bonded to a hydroxyl group directly, then it can be referred to as phenol.
Let’s trace out the required reaction:
Step 1: This is the simplest approach, in which two equivalents of Phenol reacts with acetone, catalyzed by strong acid such as sulphuric acid or hydrochloric acid, gives Bisphenol A:
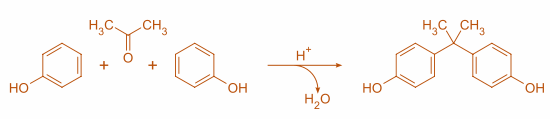
Here in the above reaction, the acid protonates the acetone and then 2 molecules of phenol interact with one mole of the protonated acetone and then come together and make a bond followed by removal of $ {H_2}O $ .
So clearly, we can conclude that the correct answer is Option B.
Note :
There are so many industrial uses of phenol, Phenol is so cheap it attracts plenty of small-scale applications. This compound is a part of industrial paint strippers used for removal of epoxy, polyurethane, and other chemically resistant coatings in the aviation industry.
Complete Step By Step Answer:
Before jumping to the solution, let’s recall the basic concepts about Phenol and its reaction:
As we know that, Phenol consists of a hydroxyl group and phenyl group attached to each other. It dissolves in water. Earlier it was used as carbolic soap. It is mildly acidic and is corrosive to the respiratory tract, eyes, and skin.
Phenol is a crystalline solid white in color and needs to be handled with care as it can cause chemical burns. It is also known as phenolic acid. If a compound consists of a six-membered aromatic ring and bonded to a hydroxyl group directly, then it can be referred to as phenol.
Let’s trace out the required reaction:
Step 1: This is the simplest approach, in which two equivalents of Phenol reacts with acetone, catalyzed by strong acid such as sulphuric acid or hydrochloric acid, gives Bisphenol A:

Here in the above reaction, the acid protonates the acetone and then 2 molecules of phenol interact with one mole of the protonated acetone and then come together and make a bond followed by removal of $ {H_2}O $ .
So clearly, we can conclude that the correct answer is Option B.
Note :
There are so many industrial uses of phenol, Phenol is so cheap it attracts plenty of small-scale applications. This compound is a part of industrial paint strippers used for removal of epoxy, polyurethane, and other chemically resistant coatings in the aviation industry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




