
Phenol undergoes electrophilic substitution more easily than benzene because:
A) –OH group exhibits +M effect and hence increases the electron density on the o- and p- positions.
B) Oxocation is more stable than the carbocation.
C) Both (a) and (b)
D) –OH group exhibits acidic character.
Answer
520.5k+ views
Hint: Electrophilic substitution reactions are the reactions where the hydrogen atom aromatic ring is replaced by an electrophile. Hydroxyl group in phenol is an electron-donating group so it activates the aromatic ring.
Complete step by step answer:
The structures of benzene and phenol are as follows:
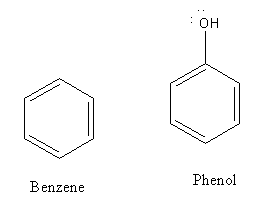
Electron deficient species is known as an electrophile. The substitution of the hydrogen atom of the aromatic ring by electrophile is known as electrophilic substitution reaction.
The rate of electrophilic substitution reaction depends on the nature of the substituent. Electron donating group increases the electron density at ortho and para position of a benzene ring and so increases the rate of electrophilic substitution reaction.
In the case of phenol, there is an interaction between the lone pair of an oxygen atom and delocalized electrons in the aromatic ring. This effect is known as the mesomeric effect or +M effect.
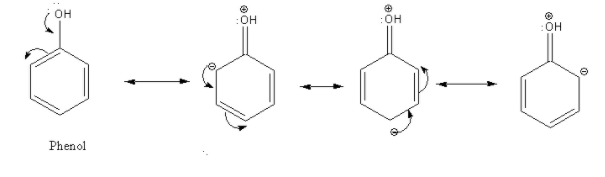
Thus, due to the electron-donating effect of the hydroxyl group in phenol, electron density increases at the ortho and para position of the aromatic ring. So, phenol undergoes electrophilic substitution more easily than benzene.
Another factor is the stability of intermediate ions.
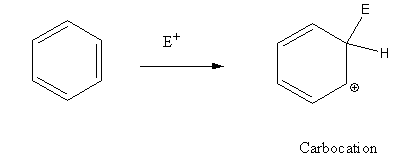
In the electrophilic substitution reaction of benzene a carbonium ion formed as an intermediate.
In electrophilic substitution reaction of phenol an oxonium ion formed as intermediate.
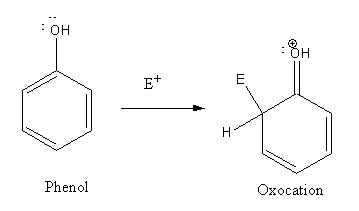
As oxocation is more stable than carbocation so phenol undergoes electrophilic substitution more easily than benzene.
Thus, the correct option is (C) both (a) and (b).
Note: Electron donating substituent is a ring activating group that increases the rate of electrophilic substitution at ortho and para position. Also due to the formation of oxocation as an intermediate phenol undergo electrophilic substitution reaction more easily than benzene.
Complete step by step answer:
The structures of benzene and phenol are as follows:

Electron deficient species is known as an electrophile. The substitution of the hydrogen atom of the aromatic ring by electrophile is known as electrophilic substitution reaction.
The rate of electrophilic substitution reaction depends on the nature of the substituent. Electron donating group increases the electron density at ortho and para position of a benzene ring and so increases the rate of electrophilic substitution reaction.
In the case of phenol, there is an interaction between the lone pair of an oxygen atom and delocalized electrons in the aromatic ring. This effect is known as the mesomeric effect or +M effect.

Thus, due to the electron-donating effect of the hydroxyl group in phenol, electron density increases at the ortho and para position of the aromatic ring. So, phenol undergoes electrophilic substitution more easily than benzene.
Another factor is the stability of intermediate ions.
In the electrophilic substitution reaction of benzene a carbonium ion formed as an intermediate.

In electrophilic substitution reaction of phenol an oxonium ion formed as intermediate.

As oxocation is more stable than carbocation so phenol undergoes electrophilic substitution more easily than benzene.
Thus, the correct option is (C) both (a) and (b).
Note: Electron donating substituent is a ring activating group that increases the rate of electrophilic substitution at ortho and para position. Also due to the formation of oxocation as an intermediate phenol undergo electrophilic substitution reaction more easily than benzene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




