
Phenolphthalein is a:
(A) Strong acid
(B) Weak acid
(C) (A) and (B) both
(D) None of the above
Answer
584.7k+ views
Hint: Phenolphthalein is an organic compound that is used as a laboratory agent and as a pH indicator. It is used as an indicator in acid-base titrations.
Complete step by step solution:
Phenolphthalein is a chemical compound having the chemical formula ${C}_{20}{H}_{14}{O}_{4}$ and is often written as PHP in short notation. It belongs to the phthalein family which is mostly used as an acid-base indicator. Because it indicates the pH of the solution, it is colourless below 8.5 pH and is able to attain a pink to deep red colour above 9.0 pH.
Phenolphthalein is a weak acid and it dissociates in water to give pink anions.
Let us see the reaction given below:
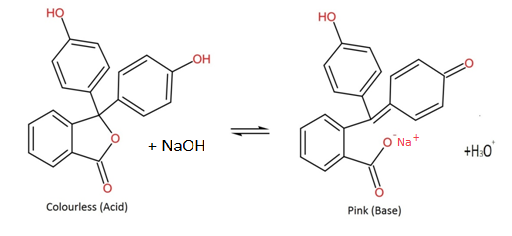
Under the acidic conditions, the equilibrium of the reaction shifts to the left and the concentration of the anions is far too low to observe the pink colour. However, when the reactions are conducted under alkaline conditions, the equilibrium shifts to the right and the concentration of the anions increases or becomes sufficient to observe the pink colour.
Hence, we can say that phenolphthalein is a weak acid.
Therefore, (B) is the correct answer.
Note: Phenolphthalein is water-soluble and can also dissolve in alcohols and this property is used for experiments. It is used to indicate the completion of an acid-base reaction. It is also used in sensors, semiconductors and in detecting viable cells. It is also used as a method to prevent drug misuse.
Complete step by step solution:
Phenolphthalein is a chemical compound having the chemical formula ${C}_{20}{H}_{14}{O}_{4}$ and is often written as PHP in short notation. It belongs to the phthalein family which is mostly used as an acid-base indicator. Because it indicates the pH of the solution, it is colourless below 8.5 pH and is able to attain a pink to deep red colour above 9.0 pH.
Phenolphthalein is a weak acid and it dissociates in water to give pink anions.
Let us see the reaction given below:

Under the acidic conditions, the equilibrium of the reaction shifts to the left and the concentration of the anions is far too low to observe the pink colour. However, when the reactions are conducted under alkaline conditions, the equilibrium shifts to the right and the concentration of the anions increases or becomes sufficient to observe the pink colour.
Hence, we can say that phenolphthalein is a weak acid.
Therefore, (B) is the correct answer.
Note: Phenolphthalein is water-soluble and can also dissolve in alcohols and this property is used for experiments. It is used to indicate the completion of an acid-base reaction. It is also used in sensors, semiconductors and in detecting viable cells. It is also used as a method to prevent drug misuse.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




